
Structure of Class I MHC Molecules
 المؤلف:
Abbas, A. K., Lichtman, A. H., & Pillai, S
المؤلف:
Abbas, A. K., Lichtman, A. H., & Pillai, S
 المصدر:
Basic Immunology : Function and disorders of immune system
المصدر:
Basic Immunology : Function and disorders of immune system
 الجزء والصفحة:
6th ed , page 58-59
الجزء والصفحة:
6th ed , page 58-59
 2025-01-05
2025-01-05
 1012
1012
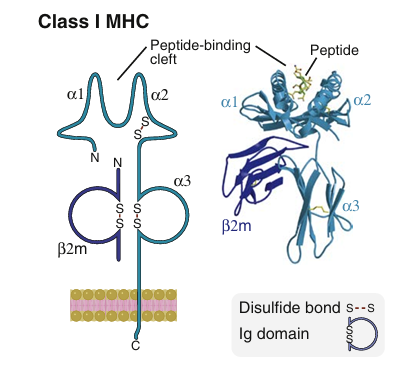
Each class I MHC molecule consists of an α chain noncovalently associated with a protein called β2-microglobulin that is encoded by a gene outside the MHC. The α chain consists of three extracellular domains followed by transmembrane and cytoplasmic domains.
• The amino-terminal αl and α2 domains of the α chain form two walls and a peptide-binding cleft, or groove, that can accommodate peptides typically 8 to 9 amino acids long. The floor of the peptide-binding cleft contains amino acid residues that bind peptides for display to T lymphocytes, and the tops of the cleft
walls make contact with the T cell receptor (which also contacts part of the displayed peptide) . The polymorphic residues of class I molecules—that is, the amino acids that differ among different individuals' MHC molecules are located in the αl and α2 domains of the α chain. Most of these polymorphic residues contribute to variations in the floor of the peptide-binding cleft and thus influence the ability of different MHC molecules to bind distinct sets of peptides.
The α3 domain is invariant and contains a site that binds the CD8 T cell coreceptor but not CD4., T cell activation requires recognition of MHC-associated peptide antigen by the TCR and simultaneous recognition of the MHC molecule by the coreceptor. Therefore, CD8+ T cells can only respond to peptides displayed by class I MHC molecules, the MHC molecules to which the CD8 coreceptor binds.
 الاكثر قراءة في المناعة
الاكثر قراءة في المناعة
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


