

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
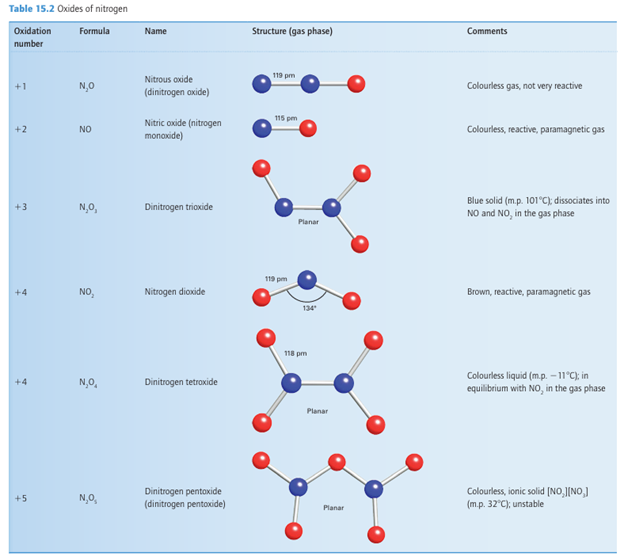
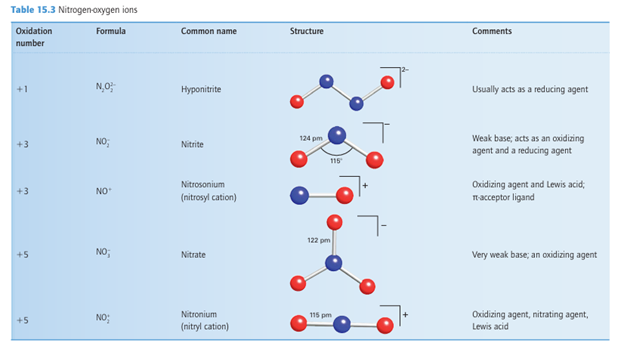
Nitrogen(V) oxides and oxoanions
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص387-389
2025-09-08
69
Nitrogen(V) oxides and oxoanions
Key points: The nitrate ion is a strong but slow oxidizing agent at room temperature; strong acid and heating accelerate the reaction.
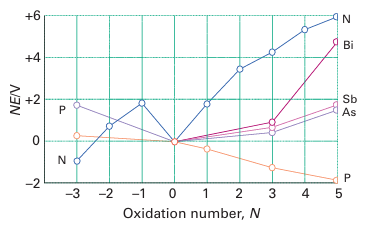
Figure 15.6 Frost diagram for the elements of the nitrogen group in acidic solution. The species with oxidation number 3 are NH3, PH3, and AsH3, and those with oxidation numbers 2 and 1 are N2H4 and NH2OH, respectively. The positive oxidation states refer to the most stable oxo or hydroxo species in acidic solution, and may be oxides, oxoacids, or oxoanions.
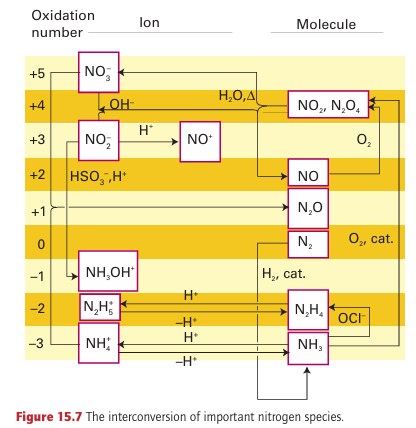
The most common source of N(V) is nitric acid, HNO3, which is a major industrial chemical used in the production of fertilizers, explosives, and a wide variety of nitrogen-containing chemicals. It is produced by modern versions of the Ostwald process, which make use of an indirect route from N2 to the highly oxidized com pound HNO3 via the fully reduced compound NH3 . Thus, after nitrogen has been reduced to the -3 state as NH3 by the Haber process, it is oxidized to the +4 state:
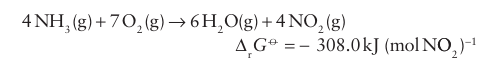
The NO2 then undergoes disproportionation into N(II) and N(V) in water at elevated temperatures:
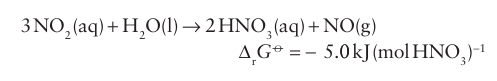
All the steps are thermodynamically favourable. The byproduct NO is oxidized with O2 to NO2 and recirculated. Such an indirect route is used because the direct oxidation of N2 to NO2 is thermo dynamically unfavourable, with ∆r GO (NO2, g) = +51 kJ mol 1. In part, this endergonic character is due to the great strength of the N≡N bond. Standard potential data imply that the NO3 ion is a mod erately strong oxidizing agent. However, its reactions are generally slow in dilute acid solution. Because protonation of an O atom promotes NO bond breaking, concentrated HNO3 (in which NO3 is protonated) undergoes more rapid reactions than the dilute acid (in which HNO3 is fully deprotonated). It is also a thermodynamically more potent oxidizing agent at low pH. A sign of this oxidizing character is the yellow colour of the concentrated acid, which indicates its instability with respect to decomposition into NO2:
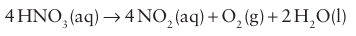
This decomposition is accelerated by light and heat. The reduction of NO-3 ions rarely yields a single product, as so many lower oxidation states of nitrogen are available. For example, a strong reducing agent such as zinc can reduce a substantial proportion of dilute HNO3 as far as oxidation state 3:
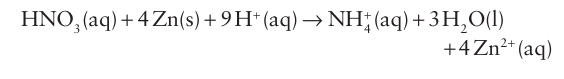
A weaker reducing agent, such as copper, proceeds only as far as oxidation state +4 in the concentrated acid:
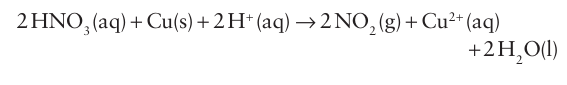
With the dilute acid, the +2oxidation state is favoured, and NO is formed:
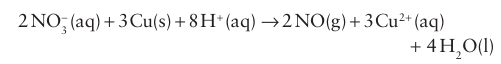
Aqua regia is a mixture of concentrated nitric acid and concentrated hydrochloric acid, which is yellow due to the presence of the decomposition products NOCl and Cl2. It loses its potency as these volatile products are formed:
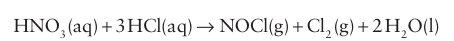
Aqua regia is Latin for ‘royal water’ and was so called by alchemists because of its ability to dissolve the noble metals gold and platinum. Gold will dissolve to a very small extent in concentrated nitric acid. In aqua regia the Cl ions present react immediately
with the Au3 ions formed to produce [AuCl4]- and thereby remove Au3 from the product side of the oxidation reaction:
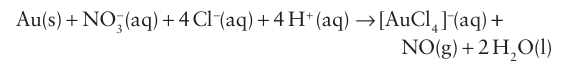
The anhydride of nitric acid is N2O5. It is a crystalline solid with the more accurate formula [NO2+] [NO2−] and can be prepared by dehydration of nitric acid with P4O10:
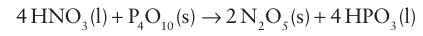
Thesolid sublimes at 320ºC and the gaseous molecules dissociate to give NO2 and O2. The compound is a strong oxidizing agent and can be used for the synthesis of anhydrous nitrates:
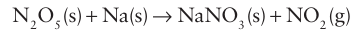

 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















