
Cooperative magnetism
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص502-504
الجزء والصفحة:
ص502-504
 2025-09-29
2025-09-29
 319
319
Cooperative magnetism
Key point: In solids, the spins on neighbouring metal centres may interact to produce magnetic behaviour, such as ferromagnetism and antiferromagnetism, that are representative of the whole solid. In the solid state the individual magnetic centres are often close together and separated by only a single atom, typically O. In such arrays cooperative properties can arise from interactions between electron spins on different atoms.
The magnetic susceptibility, x, of a material is a measure of how easy it is to align electron spins with the applied magnetic field in the sense that the induced magnetic moment is proportional to the applied field, with the constant of proportionality. A paramagnetic material has a positive susceptibility and a diamagnetic material has a negative susceptibility. Magnetic effects arising from cooperative phenomena can be very much larger than those arising from individual atoms and ions. The susceptibility and its variation with temperature are different for different types of magnetic materials and are summarized in Table 20.11 and Fig. 20.37. The application of a magnetic field to a paramagnetic material result in the partial alignment of the spins parallel to the field. As a paramagnetic material is cooled, the dis ordering effect of thermal motion is reduced, more spins become aligned, and the magnetic susceptibility increases. In a ferromagnetic substance, which is one example of a cooperative magnetic property, the spins on different metal centres are coupled into a parallel alignment that is sustained over thousands of atoms to form a magnetic domain (Fig. 20.38). The net magnetic moment, and hence the magnetic susceptibility, may be
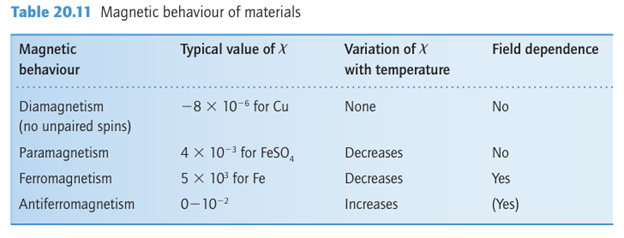
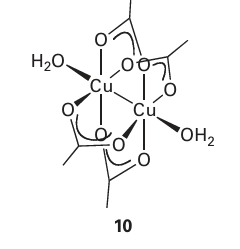
very large because the magnetic moments of individual spins add to each other. Moreover, once established and with the temperature maintained below the Curie temperature (TC), the magnetization persists after the applied field is removed because the spins are locked together. Ferromagnetism is exhibited by materials containing unpaired electrons in d or, more rarely, f orbitals that couple with unpaired electrons in similar orbitals on surround ing atoms. The key feature is that this interaction is strong enough to align spins but not so strong as to form covalent bonds, in which the electrons would be paired. At temperatures above TC the disordering effect of thermal motion overcomes the ordering effect of the interaction and the material becomes paramagnetic (Fig. 20.37). The magnetization, M, of a ferromagnet, its bulk magnetic moment, is not propor tional to the applied field strength H. Instead, a ‘hysteresis loop’ is observed like that shown in Fig. 20.39. For hard ferromagnets the loop is broad and M remains large when the applied field has been reduced to zero. Hard ferromagnets are used for permanent magnets where the direction of the magnetization does not need to be reversed. A soft ferromagnet has a narrower hysteresis loop and is therefore much more responsive to the applied field. Soft ferromagnets are used in transformers, where they must respond to a rapidly oscillating field. In an antiferromagnetic material, neighbouring spins are locked into an antiparallel alignment (Fig. 20.40). As a result, the collection of individual magnetic moments cancel and the sample has a low magnetic moment and magnetic susceptibility (tending, in fact, to zero). Antiferromagnetism is often observed when a paramagnetic material is cooled to a low temperature and is indicated by a sharp decrease in magnetic susceptibility at the Néel temperature, TN (Fig. 24.37). Above TN the magnetic susceptibility is that of a para magnetic material, and decreases as the temperature is raised. The spin coupling responsible for antiferromagnetism generally occurs through inter vening ligands by a mechanism called superexchange. As indicated in Fig. 20.41, the spin on one metal atom induces a small spin polarization on an occupied orbital of a ligand, and this spin polarization results in an antiparallel alignment of the spin on the adjacent metal atom. This alternating …↑↓↑↓… alignment of spins then propagates throughout the material. Many d-metal oxides exhibit antiferromagnetic behaviour that can be ascribed to a superexchange mechanism involving O atoms, for example MnO is antiferromagnet ic below 122 K and Cr2 O3 is antiferromagnetic below 310 K. Coupling of spins through intervening ligands is frequently observed in molecular complexes containing two ligand bridged metal ions but it is weaker than with a simple O2 link between metal sites and as a result the ordering temperatures are much lower, typically below 100 K. In ferrimagnetism, a net magnetic ordering of ions with different individual magnetic moments is observed below the Curie temperature. These ions can order with opposed spins, as in antiferromagnetism, but because the individual spin moments are different, there is incomplete cancellation and the sample has a net overall moment. As with antiferro-magnetism, these interactions are generally transmitted through the ligands; an example is magnetite Fe3O4. There are a large number of molecular systems where magnetic coupling is observed. Typical systems have two or more metal atoms bridged by ligands that mediate the coupling. Simple examples include copper acetate (10), which exists as a dimer with antiferromagnetic coupling between the two d9 centres. Many metalloenzymes (Sections 27.9 to 27.14) have multiple metal centres that show magnetic coupling.
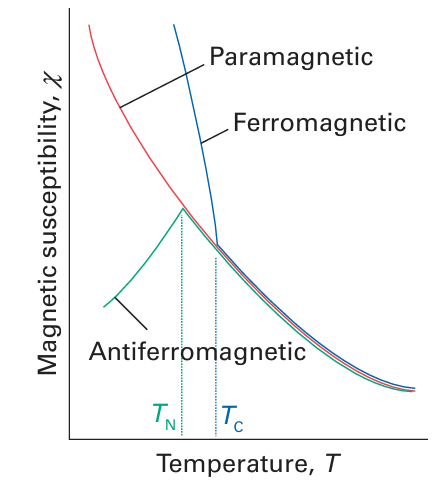
Figure 20.37 The temperature dependence of the susceptibilities of paramagnetic, ferromagnetic, and antiferromagnetic substances.
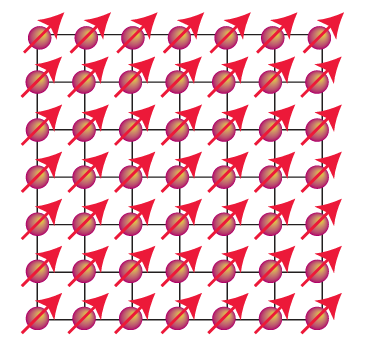
Figure 20.38 The parallel alignment of individual magnetic moments in a ferromagnetic material.
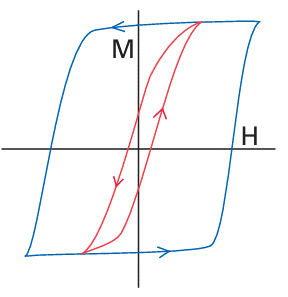
Figure 20.39 Magnetization curves for ferromagnetic materials. A hysteresis loop results because the magnetization of the sample with increasing field (→) is not retraced as the field is decreased (←). Blue line: hard ferromagnet; red line: soft ferromagnet.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


