

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Chemical vapour deposition
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
665
2025-10-14
51
Chemical vapour deposition
Key point: In chemical vapour deposition methods, a vapour of molecules chemically interact or decompose at or near the substrate, where they adsorb on the surface and combine with other species to create a solid and residual gaseous product. The control over complex stoichiometries, the ability to achieve monolayer-by-monolayer growth, and the attainment of high-quality films is not limited to physical vapour techniques. Chemical techniques, such as metal/organic chemical vapour deposition (MOCVD) and atomic layer deposition (ALD), also provide these levels of control. In contrast to physical methods, in which species condense directly onto a substrate and react with one another, chemical techniques require that a precursor decomposes chemically on or near the substrate in order to deliver the reactant species to the growing film. Therefore, in chemical vapour methods, the decomposition thermodynamics of the selected precursors must be considered because the vapour often contains elements that must not be incorporated in the growing films. Typically, the kinetic energy of the arriving species is relatively low in chemical techniques, and the temperature of the substrate can be controlled externally. The layout of a typical chemical vapour deposition (CVD) system was shown Fig. 25.5. Such systems normally operate at moderate vacuum or even at atmospheric pressure (in the region of 0.1–100 kPa). Their growth rates can be quite high, more than 10 times that of MBE or PLD. In CVD techniques, chemical decomposition of the feed molecules proceeds upstream of a substrate surface on which the desired product will be grown. The decomposition of the gaseous reactants is activated by high temperatures, lasers, or plasmas. Large numbers of different materials have been grown using CVD methods. For Group 13/15 (III/V) semiconductors (such as GaAs), the typical sources are organometallic precursors for the Group 13 element (such as Ga (CH3)3), and hydrides or chlorides for the Group 15 element (such as AsH3). A typical reaction is
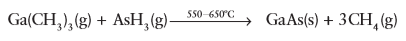
carried out in a hydrogen environment. The CVD technique can be fine-tuned to produce very high-quality films. The drawbacks of CVD include the use of toxic chemicals, turbulent flow in the reaction chamber, the incorporation of unwanted chemical species owing to incomplete decomposition or exhaust, and the use of high pressure (which prevents the use of in situ diagnostics). This same technique, however, can be used without a substrate to create nanoparticles by pyrolysis of the vapour species. Furthermore, MOCVD and MBE have been combined to allow for in situ monitoring of growth. This technique has been called chemical beam epitaxy (CBE). These low pressures decrease the interactions in the reactant stream and lead to more ballistic transport of the chemical vapour species to the substrate, where they decompose and react to form a film. A final chemical approach aims to control the precise chemical interactions that occur at a surface. In the process called atomic layer deposition (ALD), chemical species are delivered sequentially to a substrate on which a single monolayer deposits. The excess reactant is removed. Repetition of this monolayer coverage, subsequent reaction, and removal of excess reactants allows for precise control over the growth of complex materials. In this process, it is necessary to control the chemical species and their interactions to ensure monolayer coverage only and facile subsequent reaction. To do so means that the vapour of each reagent must interact in the proper manner with the film layer deposited previously. The technique produces flat, homogeneously coated layers. Some examples of ALD-grown nanomaterials are Al2O3, ZrO2, HfO2, CuS, and BaTiO3.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















