علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Inorganic–organic nanocomposites
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص678-681
2025-10-15
71
Inorganic–organic nanocomposites
Key points: Class I inorganic–organic materials have noncovalent interactions and class II have some covalent interactions; sol–gel and self-assembly methods are key chemical routes to hybrid nanocomposite design and synthesis; the properties of polymer nanocomposites are controlled through the nature of the polymeric and inorganic phases, as well as through their dispersions and interactions. Inorganic–organic nanocomposites are a third class of three-dimensional ordered materials that possess chemical and physical properties that can be tuned by using the synergistic association of organic and inorganic components at nanoscales. These hybrid materials originated in the paint and polymer industries where inorganic fillers and pigments were dispersed in organic materials (including solvents, surfactants, and polymers) to fabricate commercial products with improved materials performance. Hybrid nanomaterials are also of interest because their mechanical properties occupy a niche between glasses and polymers, so achieving enhanced strength and robustness. Hybrid nanomaterials have been reported with excellent laser efficiencies, photostability, and ultrafast photochromic response. They also can have very large second-order and third-order nonlinear optical response, which is important in frequency conversion and optical switching for telecommunications. Nanocomposites have already entered the market-place in sunscreens, fire-retardant fabrics, stain-resistant clothing, thermoplastics, water filters, and automobile parts. Examples include the use of nylon-6/montmorillonite clay nanocomposites for timing-belt covers, television screens that are coated with indigo dyes embedded in a silica/zirconia matrix, organically doped sol–gel glassware, and sol–gel-entrapped enzymes. Hybrid nanomaterials offer the materials chemist novel routes that use rational materials design to optimize structure–property relationships. Their nanoarchitectures and resulting properties depend on the chemical nature of the components and the synergy between them. A key part of the design of these hybrids is the selective tuning of the nature, extent, and accessibility of the interfaces between the inorganic and the organic building blocks.
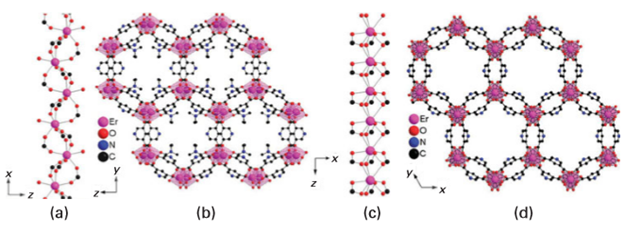
Fig 25.31 Views of the MOF structures of [Er2(PDC)3(DMF)2] ∞: (a) zigzag connection between adjacent erbium centres; (b) channels with a view along the orthorhombic x axis showing coordinated DMF molecules protruding into the channel space. Views of the structure after DMF solvent removal and rearrangement: (c) the shorter linear connection between adjacent Er3+ centres; (d) view of the open channels along the hexagonal z axis. (J. Jia, et al., Inorg. Chem., 2006, 45, 8838.) (Reproduced with permission from the American Chemical Society.) The interfaces fall into two main classes. Class I corresponds to hybrid materials where no covalent or ionic bonds are present between the organic and inorganic phases. In Class I materials, the various components interface through weak interactions such as hydrogen bonding, van der Waals contacts, and electrostatic forces. In Class II materials, at least some of the organic and inorganic components are linked through strong chemical bonds (covalent, ionic, or Lewis acid–base bonds). An important feature in the tailoring of hybrid networks is the chemical pathway used to design a given material. The main chemical routes are summarized in Fig. 25.32. It is possible to control the nature of the inorganic precursors (by using the methods described in Section 25.5), the nature of the organic material, and the method of assembling the composite material. The templated-growth fabrication processes often rely on organic molecules and macromolecules as structure-directing agents to achieve the construction of complex hierarchical architectures. It turns out that molecular and supramolecular interactions between template molecules (surfactants, amphiphilic block copolymers, organogelators, etc.) and hybrid or metal–oxo-based networks and NBBs allow a rich variety of nanocomposite materials to be prepared. Figure 25.33 illustrates two approaches to the assembly of NBBs into larger, ordered structures.
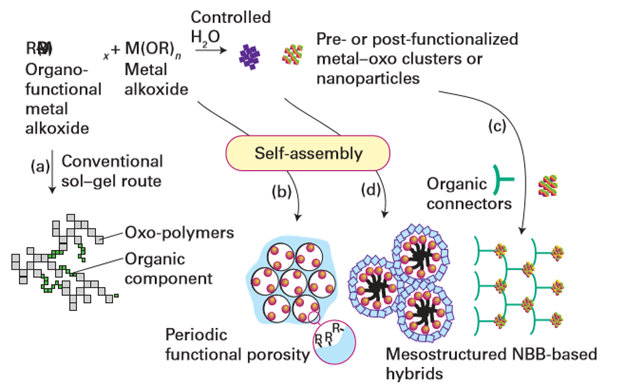
Figure 25.32 Different routes to obtain hybrid nanomaterials. Route (a) is a conventional sol–gel route that leads to ‘ordinary’ hybrids. Routes (b) and (d) involve the use of templates capable of self-assembly, giving rise to mesoorganized phases. Routes (c) and (d) involve the assembly of nanobuilding blocks (NBBs). (Adapted from C. Sanchez, et al., Chem. Mater., 2001, 13, 3061.)
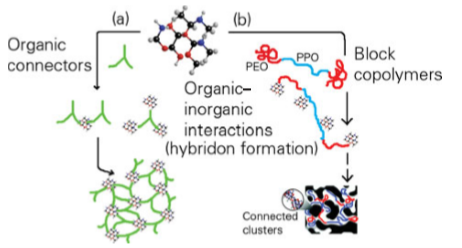
Figure 25.33 Different approaches to the assembly of nanobuilding blocks (NBBs) involving clusters and different connectors. (a) Ordered cluster dispersions based on the covalent linkage of complementary organic and inorganic NBBs. (b) Creation of a bicontinuous mesostructured hybrid arrangement. Interactions between the template and the inorganic NBB include covalent bonding and van der Waals forces (hydrophobic contacts). (Adapted from C. Sanchez, et al., Chem. Mater., 2001, 13, 3061.)
Important examples of Class I are polymer nanocomposites (PNCs). These materials are composed of inorganic nanoparticles dispersed in a polymeric matrix. Early commercial PNCs used two-dimensional ordered or lamellar clays, such as sodium montmorillonite (Na-MMT), dispersed in the polymer matrix. Such dispersants (or fillers) have sandwich-type structures (with channels between layers), a total thickness of 0.3–1 nm, and a length of 50–100 nm for each layer; these sandwich structures typically agglomerate into micrometre-sized agglomerates. The dispersion of the inorganic phase within the organic polymeric matrix in a PNC is obtained by intercalation (inserting the polymer between the layered sheets of the clay). Intercalation involves the expansion of the interlamellar spacing as a consequence of ion exchange by using organic amines or quaternary ammonium salts. Exfoliation is achieved through reactive chemical compounding or by intensive melt mixing of the clay and polymer phases. Without proper dispersion, the nanosized filler particles aggregate into larger clusters, causing degradation in the properties of the composite. Many techniques can be used to control the state of dispersion as well as the nature of the bond between nanoparticle and matrix, including the use of silanes, grafting, and CVD. Silanes are being produced that perform both tasks simultaneously: they both tailor the surface properties of the filler, which promotes the coupling of the nanoparticle to the polymer matrix, and act as a surfactant, reducing the surface energy of the filler to prevent agglomeration and promote dispersion. Radiation copolymer grafting using-rays, electron beams, or X-rays is very effective in modifying polymer surfaces. In most cases the polymer chains that are created strengthen the polymer–filler bonds. This strengthening is also evident in CVD as the gaseous molecules that form a film on the substrate typically increase the interactions between filler and polymer.
The nature of the filler (dispersant) and any cavities (voids) present in a PNC greatly influences the mechanical properties of the composites because they can control the distribution of stress through the composite matrix. Both the yield strength (a measure of the material’s ability to resist permanent deformation) and toughness (a measure of the energy absorbed prior to fracture) of nanocomposites are controlled by the nanoparticle size and dispersion and nanoparticle-to-polymer contact interactions. Therefore, control of the nanoparticle dispersion and alignment of these fillers offers a way to tailor the mechanical properties of the nanocomposites. One important class of PNCs uses nanocarbons as the dispersants. Single-walled nanotubes (SWNTs) have exceptional mechanical properties; their very high Young’s modulus (their resistance to mechanical stress), low densities, and high aspect ratios make them very attractive as fillers to enhance polymer strength. SWNTs have high tensile strength, almost 100 times that of steel, whereas their density is only about one-sixth that of steel. SWNT- reinforced composites have been developed by the physical mixing of SWNTs in solutions of preformed polymers, in situ polymerization in the presence of SWNTs, surfactant-assisted processing of SWNT–polymer composites, and chemical modification of the incorporated SWNTs. Melt processing can be carried out easily by compression moulding at high temperatures and pressures followed by rapid quenching. The so-called electrospinning methods use electrostatic forces to distort a droplet of polymer solution into a fine filament, which is then deposited on a substrate (Fig. 25.34). The nanofibres created from the electrospinning can be configured into a variety of forms including membranes, coatings, and films, and can be deposited on targets of different shapes. Fibres can be prepared with diameters smaller than 3 nm, with high surface-to-volume and length-to-diameter ratios, and with controlled pore sizes. Electrospun polymer nanocomposites have been made with homogeneously dispersed SWNTs and have exhibited significantly improved mechanical strength.
Different organo-functional groups covalently attached to the nanotubes through oxidation reactions have been used to improve their chemical compatibility with specific poly- mers (Fig. 25.35). The use of SWNTs with different functionalities allows the study of the importance of the interfacial interactions between the filler and the polymer matrix. This chemical functionalization is an effective approach towards improving the processability of the nanocomposite and the chemical compatibility of the components. As described before, specific functionalization can be used to separate the SWNT bundles and prevent their agglomeration. Metal oxide fillers have also been used to optimize polymer strengths and thermal properties. A particularly interesting example involves the control over the mechanical properties of alumina/polymethylmethacrylate (PMMA) nanocomposites by engineering the distribution of weak particle-to-polymer interactions. The filler nanoparticles
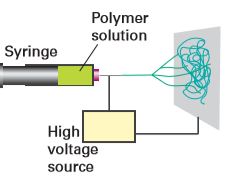
Figure 25.34 The electrospinning apparatus. (Adapted from R. Sen, et al., Nano Lett., 2004, 4, 459.)
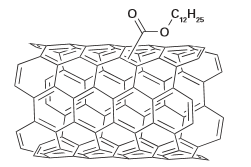
Figure 25.35 Ester-functionalized SWNTs (SWNT-COO(CH2)11CH3, EST-SWNTs). (Based on R. Sen et al., Nano Lett., 2004, 4, 459.)
(alumina) are dispersed in the composite matrix in the form of ‘net-like’ structures as opposed to ‘isolated island’ structures formed when microparticles are dispersed in the polymer matrix. In contrast to microparticle–polymer composites, these net-like structures inhibit crazing, the propagation of microscopic cracks during tensional loading. Alumina/ PMMA composites also display higher yield strengths and a shift from brittle to ductile behaviour when nanoparticulate alumina is used (Fig. 25.36). Ductility is advantageous as the composite can then be drawn into thin wires and can withstand sudden impact, thus making the composite more resilient mechanically. The addition of nano-alumina with the anti-agglomerant methacrylic acid lowers the glass transition temperature enough to change the stress–strain curve of the composite and shift from brittle to ductile under tensile loading.
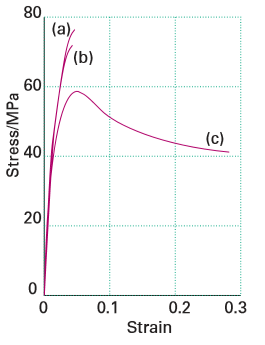
Figure 25.36 Typical stress–strain curves for (a) neat PMMA, (b) 2 per cent by mass as-received micrometre-sized alumina-filled PMMA composite, and (c) 2.2 per cent by mass 38 nm (MAA) alumina/PMMA nanocomposite. Although the strength decreases slightly for (c) (related to the decreased stress at the curve maximum), the overall ductility (related to the total strain) and toughness (related to the area under the curve) are greatly improved. (Adapted from B.J. Ash, et al., Macromolecules, 2004, 37, 1358.) (Reproduced with permission from the American Chemical Society.)
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)