علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Alkene polymerization
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص713-716
2025-10-20
62
Alkene polymerization
Key points: Heterogeneous Ziegler–Natta catalysts are used in alkene polymerization; the Cossee–Arlman mechanism describes their function; low molar mass homogeneous catalysts also catalyse the alkene polymerization reaction; considerable control over polymer tacticity is possible with judicious ligand design. Polyalkenes, which are among the most common and useful class of synthetic polymers, are most often prepared by use of organometallic catalysts, either in solution or supported on a solid surface. The development of alkene polymerization catalysts in the second half of the twentieth century, producing polymers such as polypropene and polystyrene, ushered in a revolution in packaging materials, fabrics, and constructional materials. In the 1950s J.P. Hogan and R.L. Banks discovered that chromium oxides supported on silica, a so-called Philips catalyst, polymerized alkenes to polyenes. Also in the 1950s K. Ziegler, working in Germany, developed a catalyst for ethene polymerization based on a catalyst formed from TiCl4 and Al (C2H5 )3, and soon thereafter G. Natta in Italy used this type of catalyst for the stereospecific polymerization of propene (see below). Both the Ziegler–Natta catalysts and the chromium-based polymerization catalysts are widely used today.
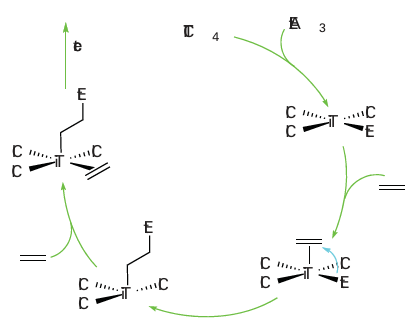
Figure 26.25 The Cossee–Arlman mechanism for the catalytic polymerization of ethene. Note that the Ti atoms are not discrete but are part of an extended structure containing bridging chlorides. The full details of the mechanism of Ziegler–Natta catalysts are still uncertain, but the Cossee–Arlman mechanism is regarded as highly plausible (Fig. 26.25). The catalyst is prepared from TiCl4 and Al(C2 H5 )3 , which react to give polymeric TiCl3 mixed with AlCl3 in the form of a fine powder. The alkylaluminium alkylates a Ti atom on the surface of the solid and an ethene molecule coordinates to the neighbouring vacant site. In the propagation steps for the polymerization, the coordinated alkene undergoes a migratory insertion reaction. This migration opens up another neighbouring vacancy, and so the reaction can continue and the polymer chain can grow. The release of the polymer from the metal atom occurs by -hydrogen elimination, and the chain is terminated. Some catalyst remains in the polymer, but the process is so efficient that the amount is negligible. The proposed mechanism of alkene polymerization on a Philips catalyst involves the initial coordination of one or more alkene molecules to a surface Cr (II) site followed by rearrange ment to metallocycloalkanes on a formally Cr(IV) site. Unlike Ziegler–Natta catalysts, the solid-phase catalyst does not need an alkylating agent to initiate the polymerization reaction; instead, this species is thought to be generated by the metallocycloalkane directly or by formation of an ethenylhydride by cleavage of a C H bond at the chromium site. Homogeneous catalysts related to the Philips and Ziegler–Natta catalysts provide additional insight into the course of the reaction and are of considerable industrial significance in their own right, being used commercially for the synthesis of specialized polymers. Most examples are from Group 4 (Ti, Zr, Hf) and are based on a bis(cyclopentadienyl) metal sys tem: the tilted ring complex [Zr(n5-Cp)2 (CH3) L] (12) is a good example. These Group 4 metallocene complexes catalyse alkene polymerization by successive insertion steps that involve prior coordination of the alkene to the electrophilic metal centre. Catalysts of this type are used in the presence of the so-called methyl aluminoxane (MAO), a poorly defined compound of approximate formula (MeAlO)n, which, among other functions, serves to methylate a starting chloride complex. Additional complications arise with alkenes other than ethene. We shall discuss only terminal alkenes such as propene and styrene, as these are relatively simple. The first com plication to consider arises because the two ends of the alkene molecule are different. In principle, it is possible for the polymer to form with the different ends head-to-head (13), head-to-tail (14), or randomly. Studies on catalysts such as (12) show that the growing chain migrates preferentially to the more highly substituted C atom of the alkene, thus giving a polymer chain that contains only head-to-tail orientations:
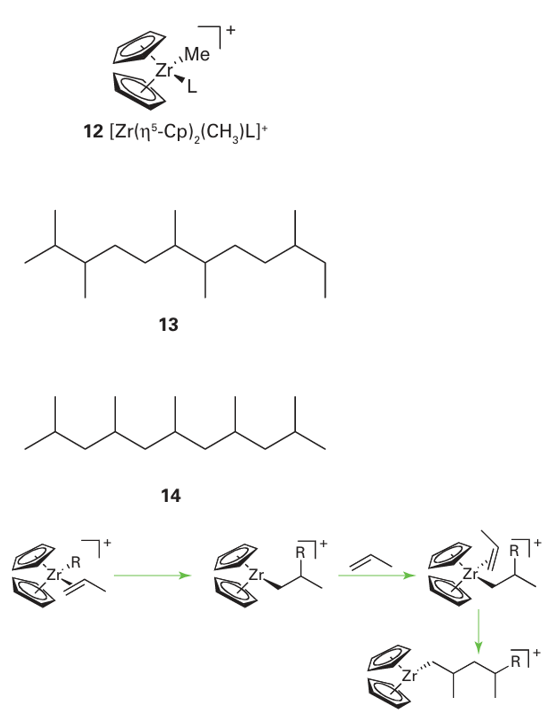
If we consider propene, we can see that the coordinated alkene induces less steric strain if its smaller CH2 end is pointing into the cleft of the (Cp)2 Zr catalyst (15), rather than the larger methyl substituted end, (16). The migrating polymer chain is thus adjacent to the methyl-substituted end of the propene molecule, and it is to this methyl-substituted C atom that the chain attaches, giving a head-to-tail sequence to the whole polymer chain. The second structural modification of polypropene is its tacticity, the relative orientations of neighbouring groups in the polymer. In a regular isotactic polypropene, all the methyl groups are on the same side of the polymer backbone (17). In regular syndiotactic polypropene, the orientation of the methyl groups alternates along the polymer chain (18). In an atactic polypropene, the orientation of neighbouring methyl groups is random (19). Control of the tacticity of a polymer is equivalent to controlling the stereospecificity of the reaction steps. The orientation of neighbouring groups is not simply of academic interest because the orientation has a significant effect on the properties of the bulk polymer. For example, the melting points of isotactic, syndiotactic, and atactic polypropene are 165ºC, 130ºC, and below 0ºC, respectively.
It is not possible to control the tacticity of polypropene with a Zr catalyst such as (15), and an atactic polymer results. However, with other catalysts it is possible to control the tacticity. The type of catalyst normally used to control the tacticity has a metal atom bond ed to two indenyl groups that are linked by a CH2CH2 bridge. Reaction of the bis(indenyl) fragment with a metal salt gives rise to three compounds: two enantiomers (20) and (21), which have C2 symmetry, and a nonchiral compound (22). These compounds are called ansa-metallocenes (the name is derived from the Latin for handle and used to indicate a bridge). It is possible to separate the two enantiomers from the nonchiral compounds and both enantiomers of these ansa-metallocenes can catalyse the stereoregular polymerization of propene. If we now consider the coordination of propene to one of the enantiomeric compounds (20) or (21) there is a second constraint in addition to the steric factor mentioned above (the CH2 group pointing towards the cleft). Of the two potential arrangements of the methyl group shown in (23) and (24), the latter is disfavoured by a steric interaction with the phenyl ring of the indenyl group. During the polymerization reaction, the R group migrates prefer entially to one side of the propene molecule; coordination of another alkene is then followed by migration, and so on. Figure 26.26 shows how an isotactic polypropene then results.
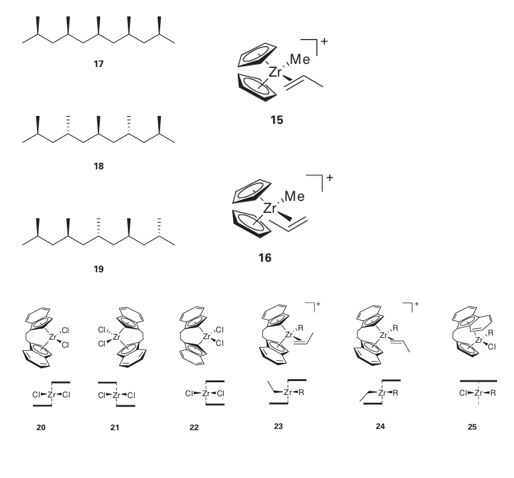
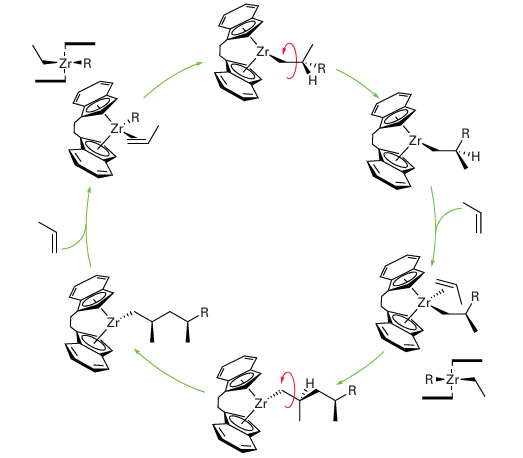
Figure 26.26 When propene is polymerized with an indenyl metallocene catalyst, isotactic polypropene results. The zirconium species all have a single positive charge; this has been omitted for clarity
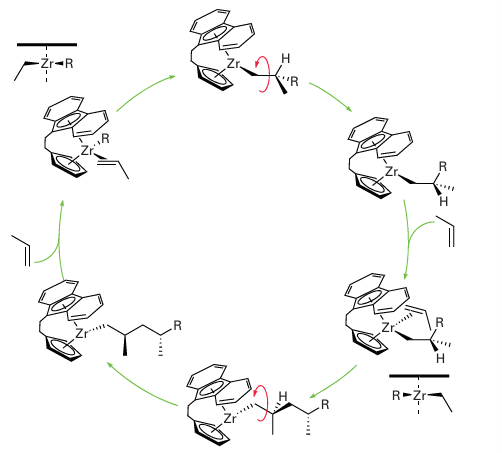
Figure 26.27 When a propene is polymerized with a fluorenyl metallocene catalyst, syndiotactic polypropene results. The zirconium species all have a single positive charge; this charge has been omitted for clarity.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)