
Biological metal-coordination sites
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص725-729
الجزء والصفحة:
ص725-729
 2025-10-22
2025-10-22
 59
59
Biological metal-coordination sites
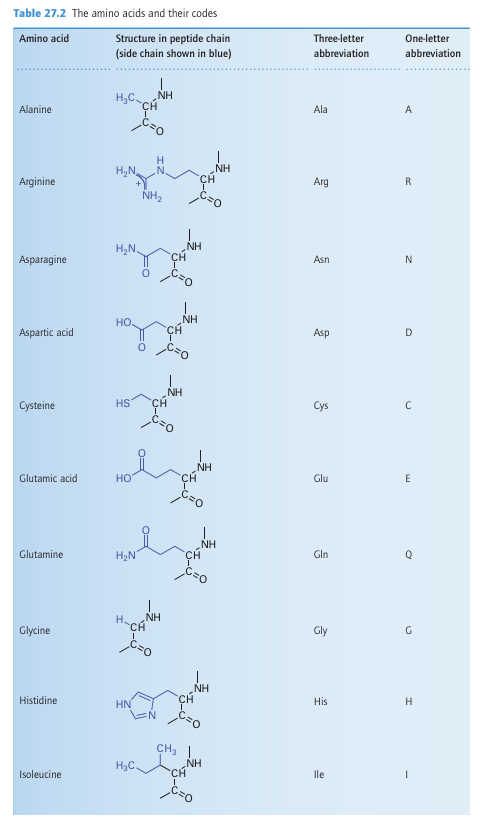
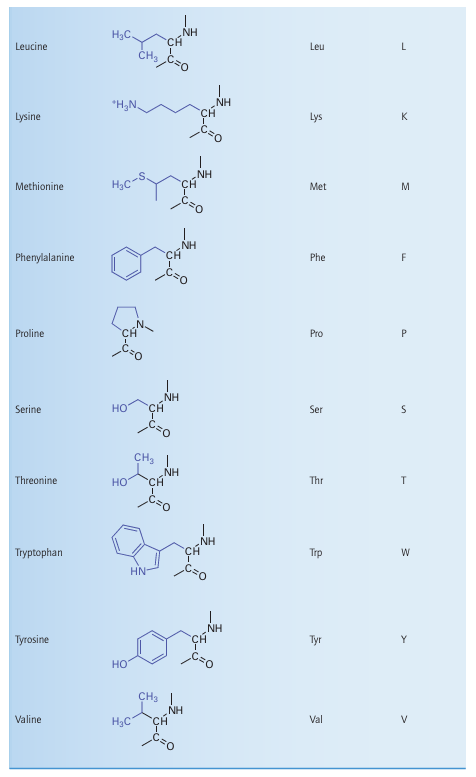
Key points: The major binding sites for metal ions are provided by the amino acids that make up protein molecules; the ligation sites range from backbone peptide carbonyls to the side chains that provide more specific complexation; nucleic acids and lipid head groups are usually coordinated to major metal ions. Metal ions coordinate to proteins, nucleic acids, lipids, and a variety of other molecules. For instance, ATP is a tetraprotic acid and is always found as its Mg2 complex (1); DNA is stabilized by weak coordination of K and Mg2 to its phosphate groups but destabilized by binding of soft metal ions such as Cu(I) to the bases. Macromolecules known as ribozymes may represent an important stage in the early evolution of life forms and are catalytic molecules composed of RNA and Mg2+. The binding of Mg+2 to phospholipid head groups is important for stabilizing membranes. There are a number of important small ligands, apart from water and free amino acids, which include sulfide, sulfate, carbonate, cyanide, carbon monoxide, and nitrogen monoxide, as well as organic acids such as citrate that form reasonably strong polydentate complexes with Fe (III). As will be familiar from introductory chemistry, a protein is a polymer with a specific sequence of amino acids linked by peptide bonds (2). A ‘small’ protein is generally regarded as one with molar mass below 20 kg mol 1, whereas a ‘large’ protein is one having a molar mass above 100 kg mol 1. The principal amino acids are listed in Table 27.2. Proteins are
synthesized, a process called translation (of the genetic code carried by DNA), on a special assembly called a ribosome. A protein may be processed further by post-translational modification, a change made to the protein structure, which includes the binding of cofactors such as metal ions. Metalloproteins, proteins containing one or more metal ions, perform a wide range of specific functions. These functions include oxidation and reduction (for which the most
important elements are Fe, Mn, Cu, and Mo), radical-based rearrangement reactions and methyl-group transfer (Co), hydrolysis (Zn, Fe, Mg, Mn, and Ni), and DNA process ing (Zn). Special proteins are required for transporting and storing different metal at oms. The action of Ca2 is to alter the conformation of a protein (its shape) as a step-in cell signalling (a term used to describe the transfer of information between and within cells). Such proteins are often known as metal ion-activated proteins. Hydrogen bonding between main-chain −NH and CO groups of different amino acids results in secondary structure (Fig. 27.2). The -helix regions of a polypeptide provide flexible mobility
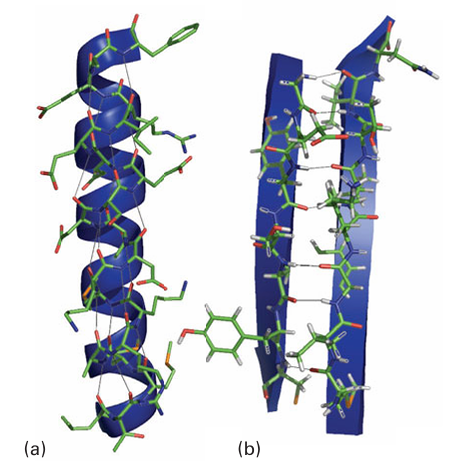
Figure 27.2 The most important regions of secondary structure, (a) helix, (b) sheet, showing hydrogen bonding between main-chain amide and carbonyl groups and their corresponding representations.
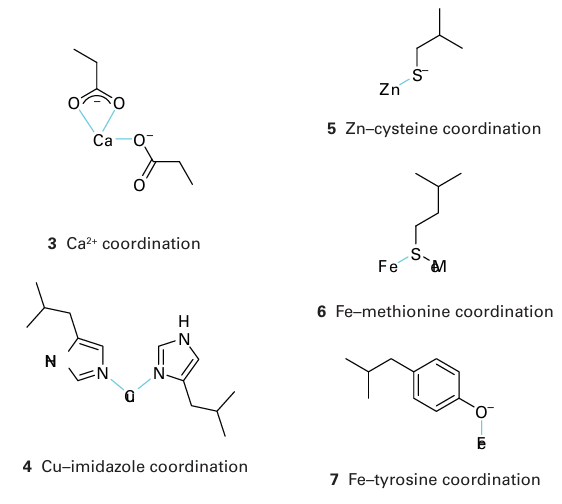
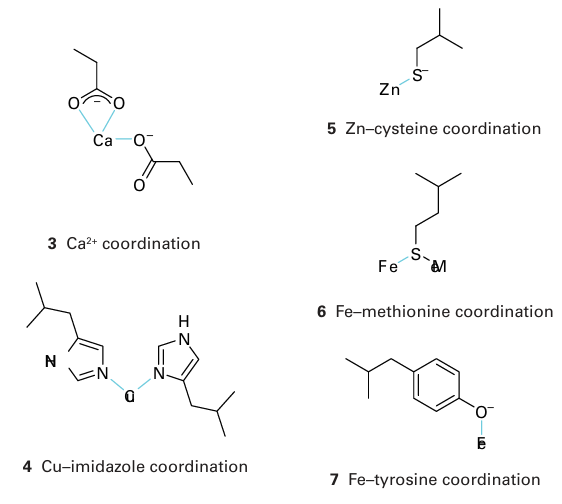
(like springs) and are important in converting processes that occur at the metal site into conformational changes; by contrast, a b-sheet region confers rigidity to support a pre organized coordination sphere suited to a particular metal ion (Sections 7.14 and 11.16). The secondary structure is largely determined by the sequence of amino acids: thus, the helix is favoured by chains containing alanine and lysine but is destabilized by glycine and proline. A protein that lacks its cofactor (such as the metal ions required for normal activity) is called an apoprotein; an enzyme with a complete complement of cofactors is known as a holoenzyme. An important factor influencing metal-ion coordination in proteins is the energy re quired to locate an electrical charge inside a medium of low permittivity. To a first ap proximation, protein molecules may be regarded as oil drops in which the interior has a much lower relative permittivity (about 4) than water (about 78). This difference leads to a strong tendency to preserve electrical neutrality at the metal site, and hence influence the redox chemistry and Brønsted acidity of its ligands. All amino acid residues can use their peptide carbonyl (or amide-N) as a donor group, but it is the side chain that usually provides more selective coordination. By referring to Table 27.2 and from the discussion in Section 4.12, we can recognize donor groups that are either chemically hard or soft and that therefore confer a particular affinity for specific metal ions. Aspartate and glutamate each provide a hard carboxylate group, and may use one or both O atoms as donors (3). The ability of Ca2 to have a high coordination number and its preference for hard donors are such that certain Ca2-binding proteins also contain the unusual amino acids -carboxyglutamate and hydroxyaspartate (generated by post-translational modification), which provide additional functionalities to enhance binding. Histidine, which has an imidazole group with two coordination sites, the ε-N atom (more common) and the -N atom, is an important ligand for Fe, Cu (4), and Zn. Cysteine has a thiol S atom that is expected to be unprotonated (thiolate) when involved in metal coordination. It is a good ligand for Fe, Cu, and Zn (5), as well as for toxic metals such as Cd and Hg. Methionine contains a soft thioether S donor that stabilizes Fe (II)(6) and Cu(I). Tyrosine can be deprotonated to provide a phenolate O donor atom that is a good ligand for Fe (III) (7). Selenocysteine (a specially coded amino acid in which Se replaces S) has also been identified as a ligand, for example it is found as a ligand to Ni in some hydrogenases (Section 27.14). A modified form of lysine, in which the side-chain −NH2 has reacted with a molecule of CO2 to produce a carbamate, is found as a ligand to Mg in the crucial photosynthetic enzyme known as rubisco (Section 27.9) and in other enzymes such as urease, where it is a ligand for Ni (II).
The primary and secondary structures of a polypeptide molecule can enforce unusual metal coordination geometries that are rarely encountered in small complexes. Protein induced strain is an important possibility, for example the protein may impose a coordination geometry on the metal ion that resembles the transition state for the particular process being executed.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


