
Oxygen atom transfer by molybdenum and tungsten enzymes
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص763-765
الجزء والصفحة:
ص763-765
 2025-10-26
2025-10-26
 53
53
Oxygen atom transfer by molybdenum and tungsten enzymes
Key points: Mo is used to catalyse O atom transfer in which the O atom is provided by a water molecule; related chemistry, but in more reducing environments, is displayed by W. Molybdenum and tungsten are the only heavier elements known so far to have specific functions in biology. Molybdenum is widespread across all life forms, and this section deals with its presence in enzymes other than nitrogenase (Section 27.13). By contrast, W has so far only been found in prokaryotes. Molybdenum enzymes catalyse the oxidation and reduction of small molecules, particularly inorganic species. Reactions include oxidation of sulfite, arsenite, xanthine, aldehydes, and carbon monoxide, and reduction of nitrate and dimethyl sulfoxide (DMSO). Both Mo and W are found in combination with an unusual class of cofactors (11), at which the metal is coordinated by a dithiolene group. In higher organisms, Mo is coordinated by one pterin dithiolene along with other ligands that often include cysteine. This is illustrated by the active site of sulfite oxidase, where we see also how the pterin ligand is twisted (48). In prokaryotes, Mo enzymes have two pterin cofactors coordinated to the metal atom and although the role of such an elaborate ligand is not entirely clear, it is potentially redox active and may mediate long-range electron transfer. Coordination of the
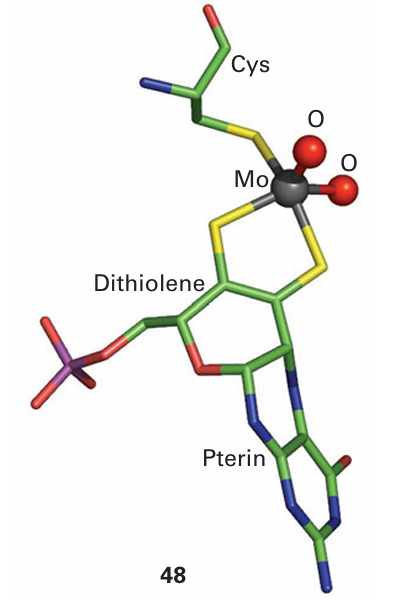
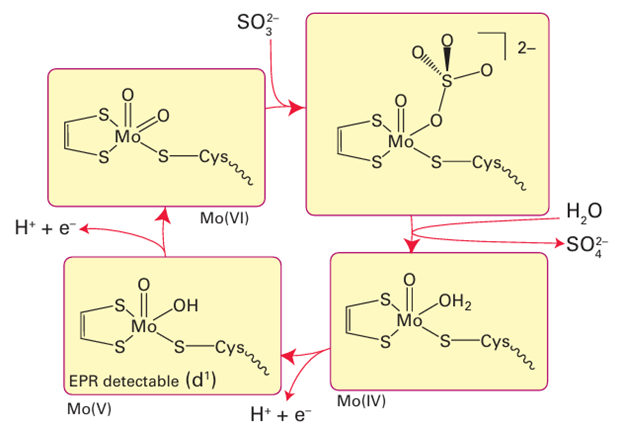
Figure 27.43 Oxidation of sulfite to sulfate by sulfite oxidase, illustrating the direct O-atom transfer mechanism for Mo enzymes.
Mo is usually completed by ligands derived from H2O, specifically H2O itself, OH, and O2. Molybdenum is suited for its role because it provides a series of three stable oxida-tion states, Mo(IV), Mo(V), and Mo(VI), related by one-electron transfers that are coupled to proton transfer. Typically, Mo(IV) and Mo(VI) differ in the number of oxido groups they contain, and Mo enzymes are commonly considered to couple one-electron transfer reactions with O-atom transfer. In humans and other mammals, an inability to synthesize molybdenum cofactor has serious consequences. Sulfite oxidase deficiency is a rare, inherited defect (sulfite ions are very toxic) and it is often fatal. A mechanism often considered for Mo enzymes is direct O-atom transfer, which is illustrated in Fig. 27.43 for sulfite oxidase. The S atom of the sulfite ion attacks an electron-deficient O atom coordinated to Mo (VI), leading to Mo–O bond cleavage, formation of Mo (IV), and dissociation of SO4 2–. Reoxidation back to Mo (VI), during which a trans ferable O atom is regained, occurs by two one-electron transfers from an Fe-porphyrin that is located on a mobile ‘cytochrome’ domain of the enzyme (Fig. 27.44). The inter mediate state containing Mo(V) (d1) is detectable by EPR spectroscopy. This kind of oxygenation reaction can be distinguished from that of the Fe and Cu enzymes described previously, because with Mo enzymes the oxido group that is trans ferred is not derived from molecular O2 but from water. The Mo (VI) O unit can trans fer an O atom, either directly (inner sphere) or indirectly to reducing (oxophilic) substrates, such as SO32– or AsO32–, but cannot oxygenate C H bonds. Figure 27.45 shows the reaction enthalpies for O-atom transfer: we see that the highly oxidizing Fe species formed by reaction with O2 are able to oxygenate all substrates, whereas Mo (VI) oxo species are limited to more reducing substrates and Mo (IV) is able to extract an O atom from nitrate. As expected from its position below Mo in Group 6, the lower oxidation states of W are less stable than those of Mo, so W(IV) species are usually potent reducing agents. This potency is illustrated by the W-containing formate dehydrogenases present in certain primitive organisms, which catalyse the reduction of CO2 to formate, the first stage in non-photosynthetic carbon assimilation. This reaction does not involve O-atom insertion but rather the formation of a C-H bond. One mechanism that has been proposed is
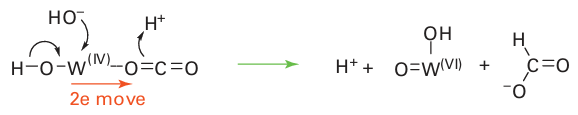
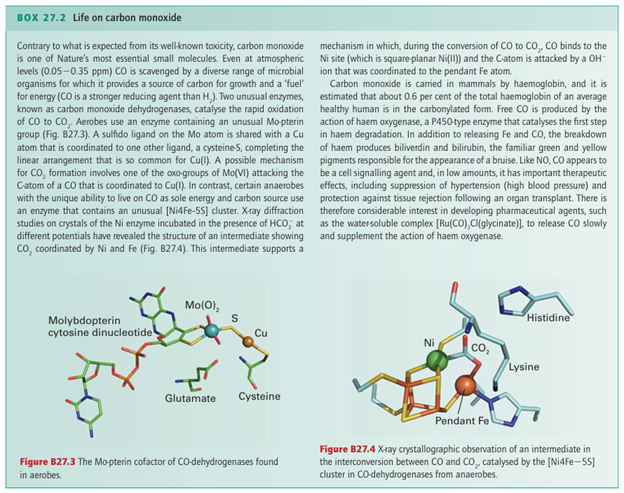
Microbial oxidation of CO to CO2 is important for removing more than 100 Mt of this toxic gas from the atmosphere every year. As shown in Box 27.2, this reaction is carried out by enzymes that contain either a Mo-pterin/Cu cofactor or an air-sensitive [Ni 4Fe 5S] cluster.
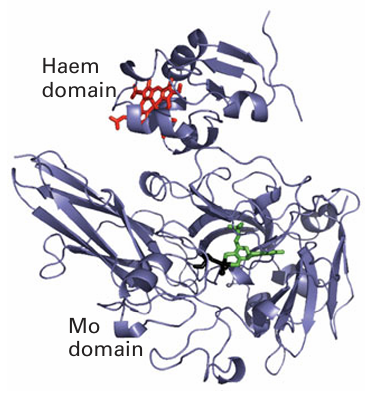
Figure 27.44 Structure of sulfite oxidase showing the Mo and haem domains. The region of polypeptide linking the two domains is highly mobile and is not resolved by crystallography.
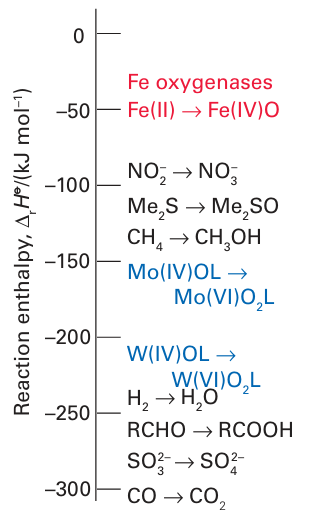
Figure 27.45 Scale showing relative enthalpies for O-atom transfer. Fe (IV) oxido species are powerful O-atom donors, whereas Mo(IV) and W(IV) are good O-atom acceptors.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


