
Isomerism in d-block metal complexes
 المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
 المصدر:
INORGANIC CHEMISTRY
المصدر:
INORGANIC CHEMISTRY
 الجزء والصفحة:
2th ed p 548
الجزء والصفحة:
2th ed p 548
 27-2-2017
27-2-2017
 1189
1189
Isomerism in d-block metal complexes
In this book so far, we have not mentioned isomerism very often, and most references have been to trans- and cis-isomers, e.g. trans-[CaI2)THF(4] and the trans- and cis-isomers of N2F2. These are geometrical isomers, and our previous discussion of this topic will not be elaborated further here.
Self-study exercises
1. Draw possible structures for the square planar complexes [PtBr2(py)2] and [PtCl3(PEt3)]-and give names to distinguish between any isomers that you have drawn.
2. In [Ru(CO)4(PPh3)], the Ru centre is in a trigonal bipyramidal environment. Draw the structures of possible isomers and give names to distinguish between them.
3. Draw the structures and name the isomers of octahedral [CrCl2(NH3)4]+.
4. Octahedral [RhCl3(H2O)3] has two isomers. Draw their structures and give them distinguishing names.
In this section, we shall be concerned with other types of isomerism exhibited by d-block metal complexes, and we use a classification that goes back to the work of Werner (Figure 1.1).
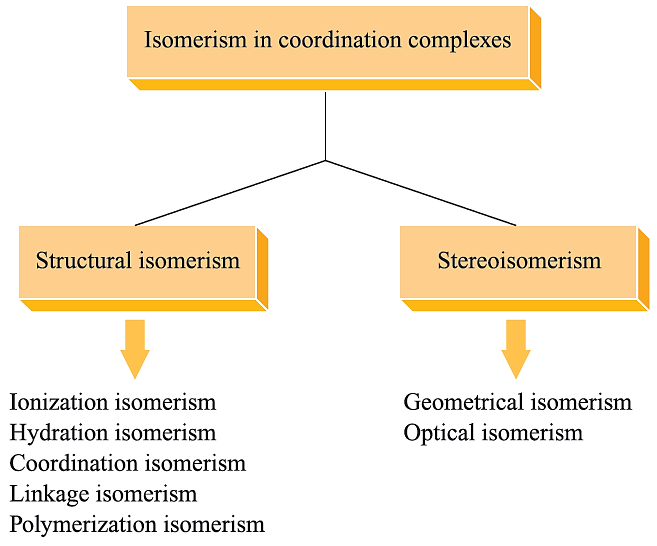
Fig. 1.1 Classification of types of isomerism in metal complexes.
 الاكثر قراءة في كيمياء العناصر الانتقالية ومركباتها المعقدة
الاكثر قراءة في كيمياء العناصر الانتقالية ومركباتها المعقدة
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


