Assay for protein content
A number of methods are available for measuring protein concentration, each being based on a specific property of proteins, and each having certain advantages and disadvantages. Consequently, the different methods are more or less suitable for different applications and it is useful to have insight into these methods so that one can decide which one to use for a given application.
1. Absorption of ultraviolet light
UV-absorption is perhaps the most simple method for measuring the concentration of proteins in solution. A typical protein absorption spectrum has an absorption peak at 280 nm, due to the aromatic amino acids, such as tryptophan and tyrosine. Below 220 nm the absorption also increases strongly, due to peptide bonds, which absorb maximally at 185 nm. The extinction coefficients of different proteins tend to be different at 280 nm, due to their different aromatic amino acid contents, while below 220 nm the extinction coefficients are more similar. It is difficult to measure absorption accurately in this part of the spectrum, however, partly because oxygen forms begins to absorb in this region.
Because the extinction coefficients of proteins differ, UV-absorption is useful as a qualitative measure, for detecting the presence of protein, but is less useful for accurate quantitative measurements, except for pure proteins of known extinction coefficient. Because of its simplicity, UV- absorption is the method favoured for continuous (semi-quantitative) monitoring of the protein concentration in the eluate from chromatography columns.
One of the limitations of UV-absorbance, as a method for measuring protein, is that UV-absorbing, non-protein, compounds may interfere with the measurement. Nucleic acids, which are ubiquitously present in biological material, absorb UV radiation strongly, with a profile overlapping that of protein, but with a maximum at 260 nm. An elegant
method for eliminating the absorption due to nucleic acids, thus allowing a measurement of protein in the presence of nucleic acid, has been proposed by Groves et al.8.
In measuring the concentration of proteins by their UV-absorbance, remember that the extinction coefficient (or absorption coefficient) is given by the equation:-
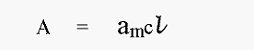
where, A = absorbance
am = molar extinction coefficient
c = molar concentration of protein in solution
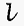 = length of the light path through the solution (usually 1 cm).
= length of the light path through the solution (usually 1 cm).
If the concentration is given in g/litre, then the equation becomes:-
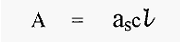
Where as= specific extinction coefficient
Note that am = as x MW.
2. The biuret assay
In alkaline solution, proteinsreduce cupric (Cu2+) ions tocuprous (Cu1+) ions which react with peptide bonds to give a blue coloured complex. This reaction is called the biuret reaction and is named after the compound biuret (I), which is thesimplestcompound thatyields the
characteristic colour.
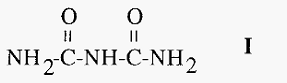
Because the reaction is with peptidebonds, thereis littlevariationin
the colour intensity given by different proteins.Thebiuret method can be used for the measurement of protein concentrationin thepresenceof
polyethylene glycol, a common protein precipitant. A disadvantage of the biuret method is thatitis relativelyinsensitive, so thatlarge amountsof proteinare required for theassay.Amore sensitive variant of the method, the micro-biuret assay, has been devised, which overcomesthis limitation tosome extent.Another limitation is that amino buffers, such as Tris, which are commonly used in the pH range ca. 8-10, can interfere with the reaction.
3. The Lowry assay
The Lowry assay may be considered as anextensionof thebiuret
assay. Initially, a copper-protein complex is formed, as in the biuret assay.The cuprous ions then reduce the so-called Folin-Ciocalteu reagent, a phosphomolybdic-phosphotungstate complex, to yield an intense blue colour.An advantageof theLowry over thebiuret assay is that it is much moresensitive, and thus consumes much less of the protein sample.A disadvantage of theLowry assay is thatitis more sensitive to interference,a consequence of themorecomplicated chemistry involved.The Lowry assay has been reviewed by Peterson.
4. The bicinchoninic acid assay
Another development of the biuret reactionis the bicinchoninic acid (BCA) assay.Bicinchoninic acidformsa 2:1complexwith cuprous ions formed in the biuret reaction, resulting in a stable, highly coloured chromophore with an absorbance maximum at 562 nm13,14. The BCA assay is more sensitive than the Lowry methodand is also less subject to
interference by a number of commonly encountered substances.As the reaction is dependent, in thefirst instance, on thereduction of cupric ions tocuprous ions by theprotein,it is sensitivetointerferenceby
strong reducing agents, e.g. ascorbic acid.Thislimitationalsoappliesto the biuret and Lowry assays.
5. The Bradford assay
A proteinassay which is rapidly becoming themostcommonly used method, due to its simplicity, sensitivity and resistance to interference,is
the dye-binding method described by Bradford. Coomassie blue G-250,
dissolved in acid solution, below pH 1, is a red-brown colour but regains its characteristic blue colour when it becomes bound toa protein.The
concentration of protein can therefore be measured by theextentto which theblue colour,measured at595nm, is restored.Coomassie blue G-250 binds largely to basic and aromatic amino acids.Different proteins will differ in their content of these amino acids and so,ideally, a standard curve should be elaborated for eachspecific protein.A modificationhas been introduced by Read and Northcote16 to overcome this problem to some extent.Adisadvantageof theBradfordassayisthatthereagent tends tostick to glass and plastic ware.For thisreason,theuse of disposable cuvettes is recommended although, if necessary, the dye can be removed from surfaces by using SDS.
References
-Dennison, C. (2002). A guide to protein isolation . School of Molecular mid Cellular Biosciences, University of Natal . Kluwer Academic Publishers new york, Boston, Dordrecht, London, Moscow .
Groves, W. E., Davis, F. C. and Sells, B. H. (1968) Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Anal. Biochem. 22, 195-210.
Lowry, O. H., Rosebrough, N. J., Farr, A. L. and Randall, R. J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265-275.
Folin, O. and Ciocalteu, V. (1927) Tyrosine and tryptophan determination in proteins. J. Biol. Chem. 73, 627-650.
Peterson, G. L. (1979) Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal. Biochem. 100, 201-220.
Smith, P. K., Krohn, R. I., Hemianson, G. T., Mallia, A. K., Gartner, F. H., Provenzano, M. D., Fugimoto, E. K., Goeke, N. M., Olsen, B. J. and Klenk, D. C. (1985) Measurement of protein using bicinchoninic acid. Anal. Biochem. 150, 76-85.
Wiechelman, K. J., Braun, R. D. and Fitzpatrick, J. D. (1988) Investigation of the bicinchoninic acid protein assay: identification of the groups responsible for color formation. Anal. Biochem. 175, 231-237.
Bradford, M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye-binding. Anal. Biochem. 72, 248-254.
Read, S. M. and Northcote, D. H. (1981) Minimization of variation in the response to different proteins of the Coomassie Blue dye-binding assay for protein. Anal. Biochem. 116, 53-64.
 الاكثر قراءة في عزل البروتين
الاكثر قراءة في عزل البروتين
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


