
Puncture
 المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
 المصدر:
A GUIDE TO PHYSICS PROBLEMS
المصدر:
A GUIDE TO PHYSICS PROBLEMS
 الجزء والصفحة:
part 2 , p 14
الجزء والصفحة:
part 2 , p 14
 3-9-2016
3-9-2016
 1614
1614
Puncture
A compressed ideal gas flows out of a small hole in a tire which has a pressure P0 inside.
a) Find the velocity of gas outside the tire in the vicinity of the hole if the flow is laminar and stationary and the pressure outside is P1.
b) Estimate this velocity for a flow of molecular hydrogen into a vacuum at a temperature T = 1000 K. Express this velocity in terms of the velocity of sound inside the tire, s0.
SOLUTION
a) Use Bernoulli’s equation for an arbitrary flow line with one point inside the tire and another just outside it. We then have
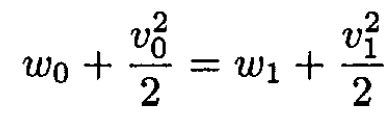 (1)
(1)
where w0 and w1 are the enthalpy per unit mass inside and outside the vessel, respectively, v0 and v1 and are the velocities of the gas. The velocity v0 is very small and can be disregarded. Then the velocity of the gas outside is
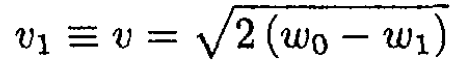 (2)
(2)
For an ideal gas the heat capacity does not depend on temperature, so we may write for the enthalpy
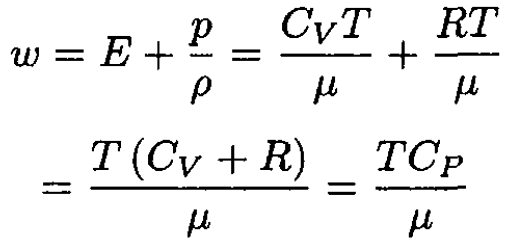 (3)
(3)
Therefore, the velocity is
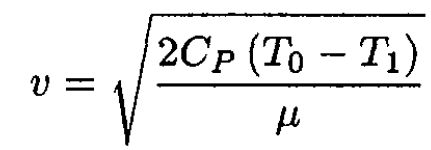 (4)
(4)
The temperature T1 may be found from the equation for adiabats and the ideal gas law:
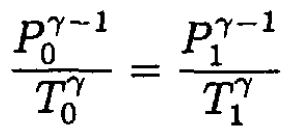 (5)
(5)
Rewriting gives
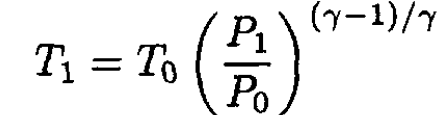 (6)
(6)
Substituting into (4) gives
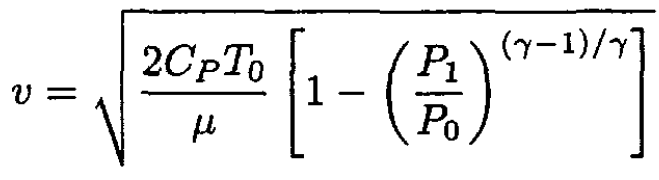 (7)
(7)
The maximum velocity vm will be reached when P1 = 0, flow into vacuum.
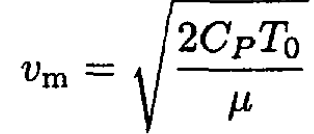 (8)
(8)
b) For one mole of an ideal gas
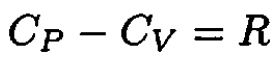 (9)
(9)
and, by definition,
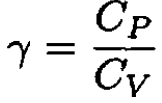 (10)
(10)
From (9) and (10), we may express CV and CP through R and γ:
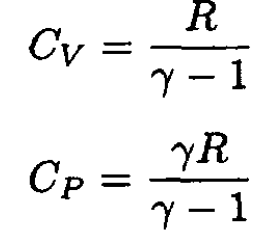 (11)
(11)
Then (8) becomes
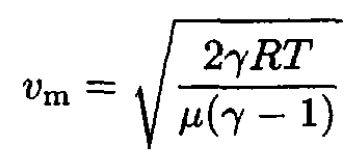 (12)
(12)
For molecular hydrogen (γ = 7/5), we have
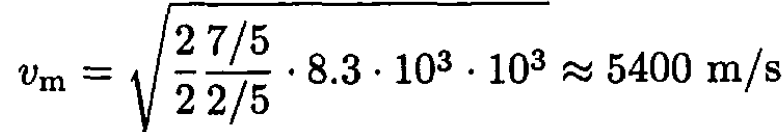
Note that this estimate implies that T1 = 0, i.e., that the gas would cool to absolute zero. This is, of course, not true; several assumptions would break down long before that. The flow during expansion into vacuum is always turbulent; the gas would condense and phase-separate and therefore would cease to be ideal. The velocity of sound inside the vessel
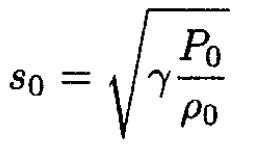 (13)
(13)
or
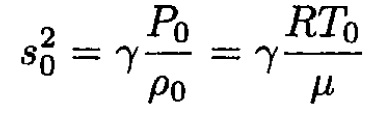 (14)
(14)
Substituting (14) into (12) yields
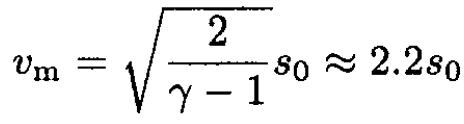 (15)
(15)
 الاكثر قراءة في مواضيع اخرى
الاكثر قراءة في مواضيع اخرى
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


