


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 19-7-2016
Date: 12-4-2017
Date: 7-8-2016
|
Entropy Increases as Crystalline Substances Dissolve
As a salt such as NaCl dissolves, the Na+ and Cl- ions leaving the crystal lattice acquire far greater freedom of motion (1.1). The resulting increase in entropy (randomness) of the system is largely responsible for the ease of dissolving salts such as NaCl in water. In thermodynamic terms, formation of the solution occurs with a favorable free-energy change: ΔG = H Δ _ TΔ S, where ΔH has a small positive value and T ΔS a large positive value; thus ΔG is negative.
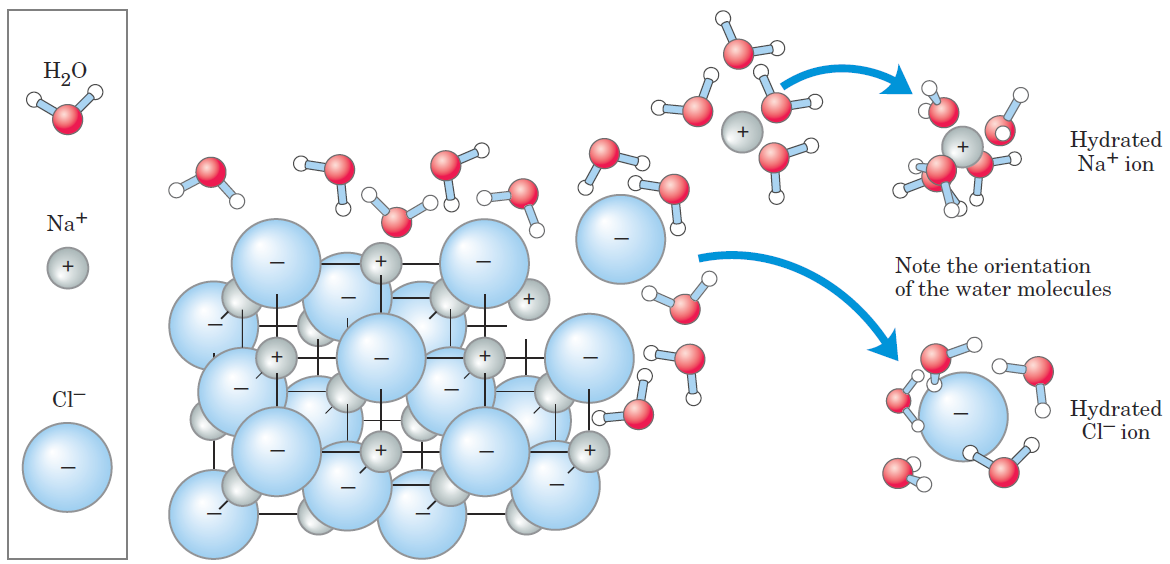
FIGURE 2–6 Water as solvent. Water dissolves many crystalline salts by hydrating their component ions. The NaCl crystal lattice is disrupted as water molecules cluster about the Cl- and Na+ ions. The ionic charges are partially neutralized, and the electrostatic attractions necessary for lattice formation are weakened.


|
|
|
|
لشعر لامع وكثيف وصحي.. وصفة تكشف "سرا آسيويا" قديما
|
|
|
|
|
|
|
كيفية الحفاظ على فرامل السيارة لضمان الأمان المثالي
|
|
|
|
|
|
|
قسم التربية والتعليم يطلق الامتحانات النهائية لمتعلِّمات مجموعة العميد التربوية للبنات
|
|
|