
Cyclohexane Conformations
 المؤلف:
John D. Roberts and Marjorie C. Caserio
المؤلف:
John D. Roberts and Marjorie C. Caserio
 المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
 الجزء والصفحة:
............
الجزء والصفحة:
............
 23-1-2022
23-1-2022
 3715
3715
Cyclohexane Conformations
If the carbons of a cyclohexane ring were placed at the corners of a regular planar hexagon, all the C−C−C bond angles would have to be 120o. Because the expected normal C−C−C bond angle should be near the tetrahedral value of 109.5o, the suggested planar configuration of cyclohexane would have angle strain at each of the carbons, and would correspond to less stable cyclohexane molecules than those with more normal bond angles. The actual normal value for the C−C−C bond angle of an open-chain −CH2−CH2−CH2− unit appears to be about 112.5o, which is 3o greater than the tetrahedral value. From this we can conclude that the angle strain at each carbon of a planar cyclohexane would be (120o−112.5o)=7.5o. Angle strain is not the whole story with regard to the instability of the planar form, because in addition to having C−C−C bond angles different from their normal values, the planar structure also has its carbons and hydrogens in the unfavorable eclipsed arrangement, as shown in Figure 12-2.
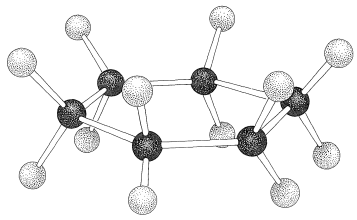
Figure 12-2: Cyclohexane in the strained planar configuration showing how the hydrogens become eclipsed.
If the carbon valence angles are kept near the tetrahedral value, you will find that you can construct ball-and-stick models of the cyclohexane six-carbon ring with two quite different conformations. These are known as the "chair" and "boat" conformations (Figure 12-3). It has not been possible to separate cyclohexane at room temperature into pure isomeric forms that correspond to these conformations, and actually the two forms appear to be rapidly interconverted. The chair conformation is considerably more stable and comprises more than 99.9% of the equilibrium mixture at room temperature.
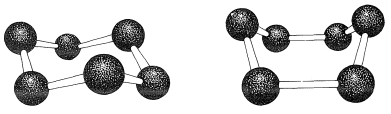
Figure 12-3: Chair (left) and boat (right) conformations of the six carbons of a cyclohexane ring with normal C−C−C bond angles.
Why is the boar form less stable than the chair form, if both have normal C−C−C bond angles? The answer is that the boat form has unfavorable nonbonded interactions between the hydrogen atoms around the ring. If we make all of the bond angles normal and orient the carbons to give the "extreme boat" conformation of Figure 12-4, a pair of 1,4 hydrogens (the so-called "flagpole" hydrogens) have to be very close together (1.83Å). Hydrogens this close together would be on the rising part of a repulsion potential energy curve, such as Figure 4-6, for hydrogen-hydrogen nonbonded interactions. This steric hindrance at an H−H distance of 1.83Å corresponds to a repulsion energy of about 3kcal mol−13kcal mol−1. There is still another factor that makes the extreme boat unfavorable; namely, that the eight hydrogens along the "sides" of the boat are eclipsed, which brings them substantially closer together than they would be in a staggered arrangement (about 2.27Å compared with 2.50Å). This is in striking contrast with the chair form (Figure 12-5), for which adjacent hydrogens are in staggered positions with respect to one another all around the ring. Therefore the chair form is expected to be more stable than the boat form because it has less repulsion between the hydrogens.
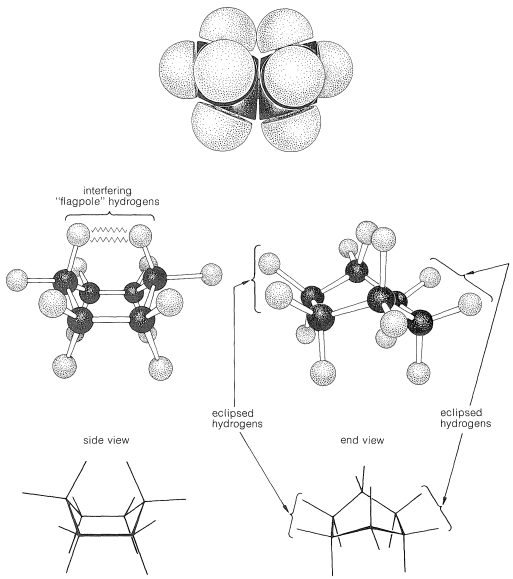
Figure 12-4: Extreme boat form of cyclohexane showing interfering and eclipsed hydrogens. Top, space-filling model; center, ball-and-stick model; bottom, sawhorse representations.
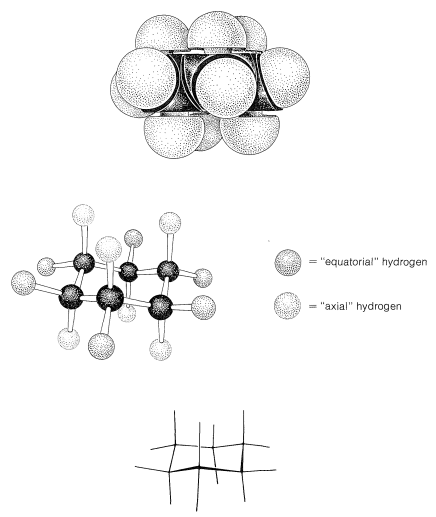
Figure 12-5: Chair form of cyclohexane showing equatorial and axial hydrogens. Top, space-filling model; center, ball-and-stick model; bottom, sawhorse representation. Notice that all the axial positions are equivalent and all the equatorial positions are equivalent. By this we mean that a substituent on any one of the six axial positions gives the same axial conformation, whereas a substituent on any one of the six equatorial positions gives the same equatorial conformation.
You should make and inspect models such as those in Figure 12-3 to see the rather striking difference between the chair and boat conformations that is not obvious from the diagrams. You will find that the chair structure is quite rigid, and rotation does not occur around the C−C bonds with interconversion to the boat structure. In contrast, the boat form is quite flexible. Rotation about the C−C bonds permits the ring to twist one way or the other from the extreme boat conformation to considerably more stable, equal-energy conformations, in which the flagpole hydrogens move farther apart and the eight hydrogens along the sides become largely but not completely staggered. These arrangements are called the twist-boat (sometimes skew-boat) conformations (see Figure 12-6) and are believed to be about 5kcal mol−1 less stable than the chair form.
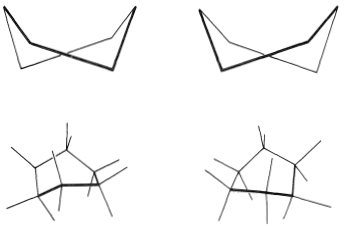
Figure 12-6: The twist-boat conformations of cyclohexane.
It is possible to measure the spectral properties of the twist-boat form by a very elegant technique employed by F. A. L. Anet. Because the equilibrium constant for conversion of chair to boat increases with temperature, a considerable proportion of the molecules exist as the twist-boat form in the vapor at 800o. If such vapor is allowed to impinge on a surface cooled to 20K, the film condensate contains about 25% of the twist-boat form. At this low temperature, the twist-boat form is converted to the more stable chair form at a very slow rate. Infrared spectra can be taken of the boat-chair mixture at 10K. If the mixture is allowed to warm to 75K, the normal equilibrium favoring the chair form is established in a short time.
 الاكثر قراءة في الهايدروكاربونات
الاكثر قراءة في الهايدروكاربونات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


