

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Hydrogenation of alkenes
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص696-698
2025-10-16
71
Hydrogenation of alkenes
Key points: Wilkinson’s catalyst, [RhCl (PPh3)3], and related complexes are used for the hydrogenation of a wide variety of alkenes at pressures of hydrogen close to 1 atm or less; suitable chiral ligands can lead to enantioselective hydrogenations. The addition of hydrogen to an alkene to form an alkane is favoured thermodynamically (∆rGO=-101 kJ mol-1 for the conversion of ethene to ethane). However, the reaction rate is negligible at ordinary conditions in the absence of a catalyst. Efficient homogeneous and heterogeneous catalysts are known for the hydrogenation of alkenes and are used in such diverse areas as the manufacture of margarine, pharmaceuticals, and petrochemicals. One of the most studied catalytic systems is the Rh(I) complex [RhCl (PPh3)3], which is often referred to as Wilkinson’s catalyst. This useful catalyst hydrogenates a wide variety of alkenes and alkynes at pressures of hydrogen close to 1 atm or less at room temperature. The dominant cycle for the hydrogenation of terminal alkenes by Wilkinson’s catalyst is shown in Fig. 26.5. It involves the oxidative addition of H2 to the 16-electron complex [RhCl (PPh3)3] (A), to form the 18-electron dihydrido complex (B). The dissociation of a
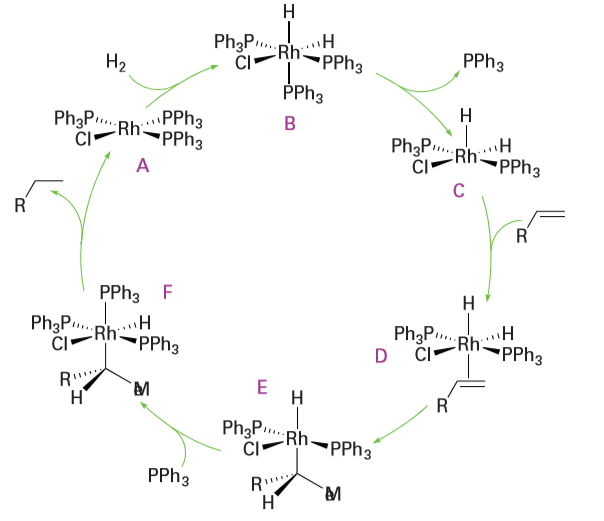
Figure 26.5 The catalytic cycle for the hydrogenation of terminal alkenes by Wilkinson’s catalyst.
phosphine ligand from (B) results in the formation of the coordinatively unsaturated complex (C), which then forms the alkene complex (D). Hydrogen transfer from the Rh atom in (D) to the coordinated alkene yields a transient 16-electron alkyl complex (E). This complex takes on a phosphine ligand to produce (F), and hydrogen migration to carbon results in the reductive elimination of the alkane and the reformation of (A), which is set to repeat the cycle. A parallel but slower cycle (which is not shown) is known in which the order of H2 and alkene addition is reversed. Another cycle is known, based around the 14-electron intermediate [RhCl (PPh3)2]. Even though there is very little of this species present, it reacts much faster with hydrogen than [RhCl (PPh3)3] and makes a significant contribution to the catalytic cycle. In this cycle, (E) would eliminate alkane directly, regenerating [RhCl (PPh3)2], which rapidly adds H2 to give (C). Wilkinson’s catalyst is highly sensitive to the nature of the phosphine ligand and the alkene substrate. Analogous complexes with alkylphosphine ligands are inactive, presumably because they are more strongly bound to the metal atom and do not readily dissociate. Similarly, the alkene must be just the right size: highly hindered alkenes or the sterically unencumbered ethene are not hydrogenated by the catalyst, presumably because the sterically crowded alkenes do not coordinate and ethene forms a strong complex that does not react further. These observations emphasize the point made earlier that a catalytic cycle is usually a delicately poised sequence of reactions, and anything that upsets its flow may block catalysis or alter the mechanism. Wilkinson’s catalyst is used in laboratory-scale organic synthesis and in the production of fine chemicals. Related Rh(I) phosphine catalysts that contain a chiral phosphine ligand have been developed to synthesize optically active products in enantioselective reactions (reactions that produce a particular chiral product). The alkene to be hydrogenated must be prochiral, which means that it must have a structure that leads to R or S chirality when complexed to the metal. The resulting complex will have two diastereomeric forms depending on which face of the alkene coordinates to the metal atom. In general, diastereomers have different stabilities and labilities, and in favourable cases one or the other of these effects leads to product enantioselectivity. Enantioselectivities are normally measured in terms of the enantiomeric excess (ee), which is defined as the percentage yield of the major enantiomeric product minus the percentage yield of the minor enantiomeric product.
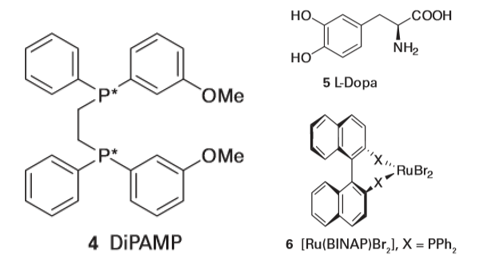
■A brief illustration. Areaction that gives 51 per cent of one enantiomer and 49 per cent of another would be described as having an enantiomeric excess of 2 per cent; a reaction that gave 99 per cent of one enantiomer and 1 per cent of another would have ee=98 per cent. An enantioselective hydrogenation catalyst containing a chiral phosphine ligand referred to as DiPAMP (4) is used to synthesize L-dopa (5), a chiral amino acid used to treatParkinson’s disease. An interesting detail of the process is that the minor diastereomer in solution leads to the major product. The explanation of the greater turnover frequency of the minor isomer lies in the difference in activation Gibbs energies (Fig. 26.6). Spurred on by clever ligand design and using a variety of metals, this field has grown rapidly and provides many clinically useful compounds; of particular note are systems derived from ruthenium (II) BINAP (6).3
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















