


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 14-8-2016
Date: 28-7-2016
Date: 3-9-2016
|
Heat Capacities
Consider a gas with arbitrary equation of state P = f (τ, V), at a temperature τ, τ < τcr, where τcr is a critical temperature of this gas.
a) Calculate CP - CV for this gas in terms of f. Does CP - CV always have the same sign?
b) Using the result of (a), calculate CP - CV for one mole of a van der Waals gas.
SOLUTION
From the definition of CP for a gas,
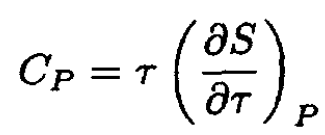 (1)
(1)
Since we are interested in a relation between CP and CV it is useful to transform to other variables than in (1), namely τ, V instead of τ, P. We will use the Jacobian transformation:
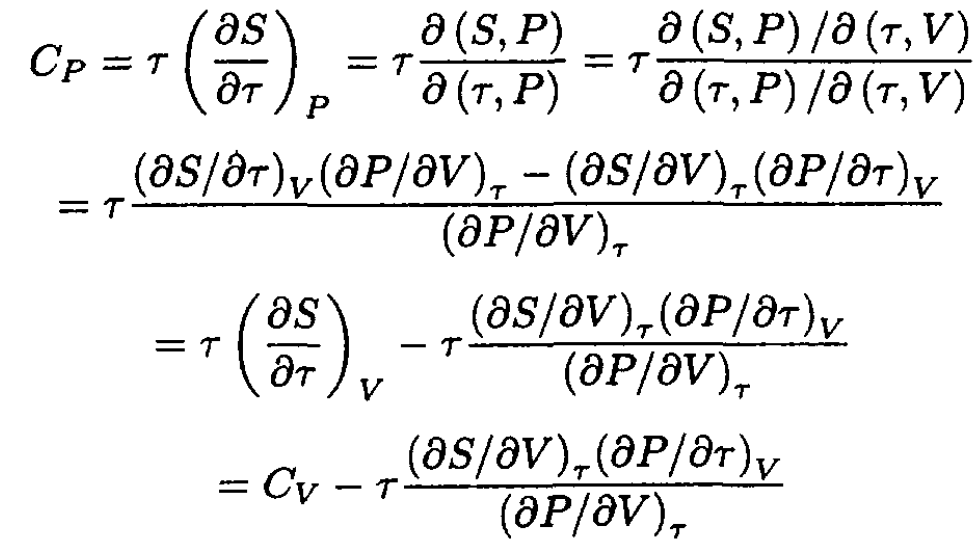 (2)
(2)
A useful identity is obtained from
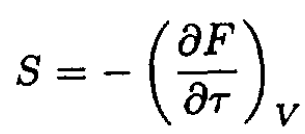 (3)
(3)
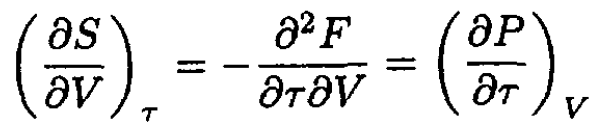 (4)
(4)
So
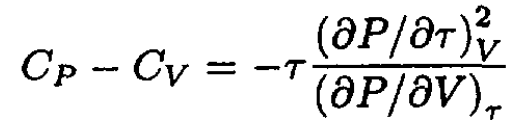 (5)
(5)
Since
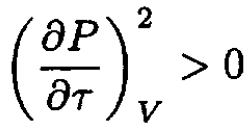
and
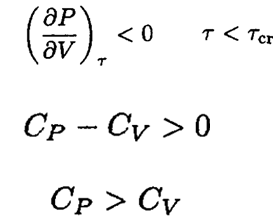
b) Let us write the van der Waals equation for one mole of the gas in the form
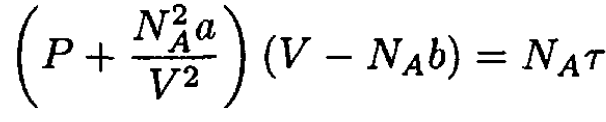 (6)
(6)
from which we obtain
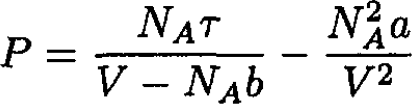 (7)
(7)
Substituting for P in (5) yields
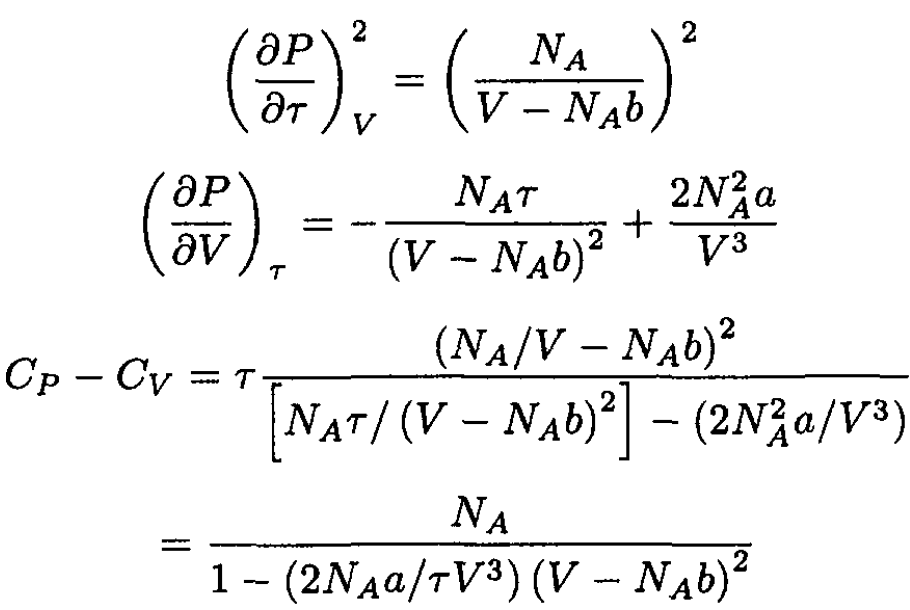
We can see that CP – CV = NA (in regular units CP – CV = R) for an ideal gas where a = b = 0.



|
|
|
|
حقن الذهب في العين.. تقنية جديدة للحفاظ على البصر ؟!
|
|
|
|
|
|
|
"عراب الذكاء الاصطناعي" يثير القلق برؤيته حول سيطرة التكنولوجيا على البشرية ؟
|
|
|
|
|
|
|
المجمع العلمي يعقد اجتماعاً لمناقشة إطلاق مشروع الدورات القرآنيّة الصيفيّة
|
|
|