


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 10-9-2020
التاريخ: 26-7-2019
التاريخ: 2-1-2022
التاريخ: 12-7-2018
|
When you meet a new reaction, you must do two things:
1. identify which bonds have been formed and broken, and
2. decide which molecule is the nucleophile and which is the electrophile. Once you have done that, you are well on the way to writing a reasonable mechanism using curly arrows. We’ll take as an example the reaction of triphenylphosphine with methyl iodide.

First observe what has happened: a new bond has been formed between the phosphorus atom and the methyl group, and the carbon–iodine bond has been broken. So, we need to draw the two reagents in such a way that a curly arrow can be used to represent this new bond. You’ll also need to make sure that you draw out all of the bonds that are actually involved in the reaction (too much detail is better than too little):
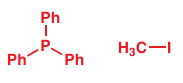
Now the all-important question: which is the nucleophile and which is the electrophile? For the nucleophile we are looking for a high-energy pair of electrons such as a lone pair, which the phosphorus has. Likewise, methyl iodide fi ts the bill as a plausible electrophile, with its bond between C and an electronegative element (I). All that remains is to draw the arrows. The fi rst one starts on the source of the electrons, the phosphorus lone pair, and finishes near the C atom to indicate the new P–C bond. The second one breaks the old C–I bond and moves electrons onto the I atom.

Admittedly, that was quite an easy mechanism to draw but you should still be pleased if you succeeded at your first try.


|
|
|
|
حقن الذهب في العين.. تقنية جديدة للحفاظ على البصر ؟!
|
|
|
|
|
|
|
"عراب الذكاء الاصطناعي" يثير القلق برؤيته حول سيطرة التكنولوجيا على البشرية ؟
|
|
|
|
|
|
|
السفارة العراقية تشيد بجناح جمعية العميد في معرض تونس الدولي للكتاب ودورها الثقافي
|
|
|