


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 16-10-2019
Date: 15-10-2019
Date: 28-10-2019
|
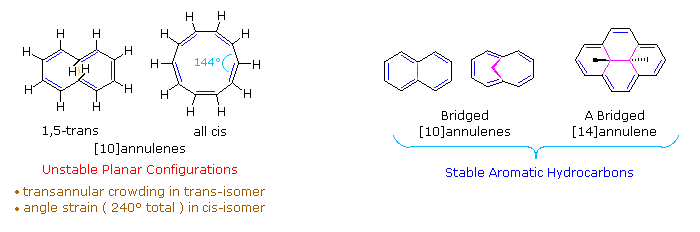
Annulenes
Monocyclic compounds made up of alternating conjugated double bonds are called annulenes. Benzene and 1,3,5,7-cyclooctatetraene are examples of annulenes; they are named [6]annulene and [8]annulene respectively, according to a general nomenclature system in which the number of pi-electrons in an annulene is designated by a number in brackets. Some annulenes are aromatic (e.g. benzene), but many are not due to non-planarity or a failure to satisfy the Hückel Rule. Compounds classified as [10]annulenes (a Hückel Rule system) serve to illustrate these factors.
As shown in the following diagram, 1,3,5,7,9-cyclodecapentaene fails to adopt a planar conformation, either in the all cis-configuration or in its 1,5-trans-isomeric form. The transannular hydrogen crowding that destabilizes the latter may be eliminated by replacing the interior hydrogens with a bond or a short bridge (colored magenta in the diagram). As expected, the resulting 10 π-electron annulene derivatives exhibit aromatic stability and reactivity as well as characteristic ring current anisotropy in the nmr. Naphthalene and azulene are [10]annulene analogs stabilized by a transannular bond. Although the CH2 bridged structure to the right of naphthalene in the diagram is not exactly planar, the conjugated 10 π-electron ring is sufficiently close to planarity to achieve aromatic stabilization. The bridged [14]annulene compound on the far right, also has aromatic properties.


|
|
|
|
حقن الذهب في العين.. تقنية جديدة للحفاظ على البصر ؟!
|
|
|
|
|
|
|
"عراب الذكاء الاصطناعي" يثير القلق برؤيته حول سيطرة التكنولوجيا على البشرية ؟
|
|
|
|
|
|
|
جمعية العميد تعقد اجتماعها الأسبوعي لمناقشة مشاريعها البحثية والعلمية المستقبلية
|
|
|