


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 2025-01-13
التاريخ: 7-2-2016
التاريخ: 2025-01-07
التاريخ: 2-11-2019
|
It is no coincidence that these and many other highly conjugated compounds are coloured. All dyes and pigments based on organic compounds are highly conjugated. The table below shows the approximate wavelengths of light absorbed by a polyene conju-gated system containing various numbers n of double bonds. Note that the colour absorbed is complementary to the colour transmitted—a red compound must absorb blue and green light to appear red.
Fewer than about eight conjugated double bonds, and the compound absorbs only in the UV. With more than eight conjugated double bonds, the absorption creeps into the visible and, by the time it reaches 11, the compound is red. Blueor green polyenes are rare, and dyes of these colours rely on more elaborate conjugated systems.
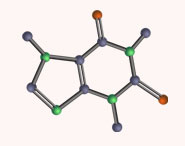
Blue jeans Transitions from bonding to antibonding π orbitals are called π→π* transitions. If electrons are instead promoted from a non-bonding lone pair (n orbital) to a π* orbital (an n→π* transition) smaller energy gaps may be available, and many dyes make use of n→π* transitions to produce colours throughout the whole spectrum. For example, the colour of blue jeans comes from the pigment indigo. The two nitrogen atoms provide the lone pairs that can be excited into the π* orbitals of the rest of the molecule. These are low in energy because of the two carbonyl groups. Yellow light is absorbed by this pigment and indigo–blue light is transmitted. Jeans are dyed by immersion in a vat of reduced indigo, which is colourless since the conjugation is interrupted by the central single bond. When the cloth is hung up to dry, the oxygen in the air oxidizes the pigment to indigo and the jeans turn blue.


|
|
|
|
مقاومة الأنسولين.. أعراض خفية ومضاعفات خطيرة
|
|
|
|
|
|
|
أمل جديد في علاج ألزهايمر.. اكتشاف إنزيم جديد يساهم في التدهور المعرفي ؟
|
|
|
|
|
|
|
العتبة العباسية المقدسة تنظّم دورةً حول آليّات الذكاء الاصطناعي لملاكاتها
|
|
|