


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 28-6-2019
Date: 31-5-2016
Date: 16-10-2020
|
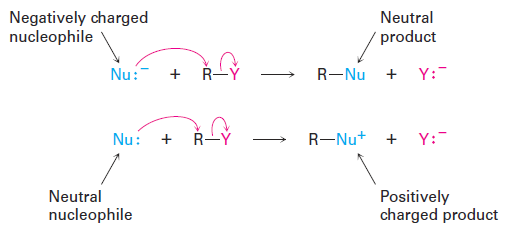
SN2 reactions-The Nucleophile
Another variable that has a major effect on the SN2 reaction is the nature of the nucleophile. Any species, either neutral or negatively charged, can act as a nucleophile as long as it has an unshared pair of electrons; that is, as long as it is a Lewis base. If the nucleophile is negatively charged, the product is neutral; if the nucleophile is neutral, the product is positively charged.
A wide array of substances can be prepared using nucleophilic substitution reactions. In fact, we’ve already seen examples in previous chapters. For instance, the reaction of an acetylide anion with an alkyl halide is an SN2 reaction in which the acetylide nucleophile displaces a halide leaving group.
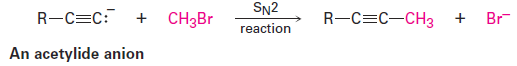
Table 1.1 lists some nucleophiles in the order of their reactivity, shows the products of their reactions with bromomethane, and gives the relative rates of their reactions. Clearly, there are large differences in the rates at which various nucleophiles react. What are the reasons for the reactivity differences observed in Table 1.1? Why do some reactants appear to be much more “nucleophilic” than others? The answers to these questions aren’t straightforward. Part of the problem is that the term nucleophilicity is imprecise. The term is usually taken to be a measure of the affinity of a nucleophile for a carbon atom in the SN2 reaction, but the reactivity of a given nucleophile can change from one reaction to the next. The exact nucleophilicity of a species in a given reaction depends on the substrate, the solvent, and even the reactant concentrations. Detailed explanations for the observed nucleophilicities aren’t always simple, but some trends can be detected from the data of Table 1.1.
Table 1.1 Some SN2 Reactions with Bromomethane
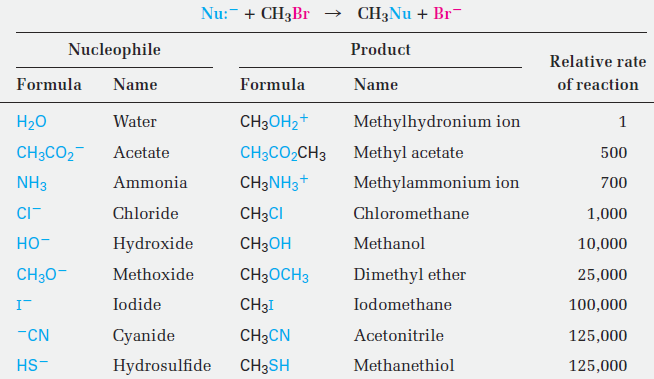
• Nucleophilicity roughly parallels basicity when comparing nucleophiles that have the same reacting atom. Thus, OH- is both more basic and more nucleophilic than acetate ion, CH3CO2-, which in turn is more basic and more nucleophilic than H2O. Since “nucleophilicity” is usually taken as the affinity of a Lewis base for a carbon atom in the SN2 reaction and “basicity” is the affinity of a base for a proton, it’s easy to see why there might be a correlation between the two kinds of behavior.
• Nucleophilicity usually increases going down a column of the periodic table. Thus, HS- is more nucleophilic than HO-, and the halide reactivity order is I- . Br- . Cl-. Going down the periodic table, elements have their valence electrons in successively larger shells where they are successively farther from the nucleus, less tightly held, and consequently more reactive. This matter is complex, though, and the nucleophilicity order can change depending on the solvent.
• Negatively charged nucleophiles are usually more reactive than neutral ones. As a result, SN2 reactions are often carried out under basic conditions rather than neutral or acidic conditions.


|
|
|
|
حقن الذهب في العين.. تقنية جديدة للحفاظ على البصر ؟!
|
|
|
|
|
|
|
"عراب الذكاء الاصطناعي" يثير القلق برؤيته حول سيطرة التكنولوجيا على البشرية ؟
|
|
|
|
|
|
|
جمعية العميد تعقد اجتماعها الأسبوعي لمناقشة مشاريعها البحثية والعلمية المستقبلية
|
|
|